Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: FcRn (the neonatal Fc receptor) is a receptor expressed by antigen-presenting cells, such as monocytes, macrophages, and dendritic cells, as well as on neutrophils. Its function is to “rescue” IgG and albumin from degradation. UCB4470 is an anti-mouse FcRn antibody that specifically blocks the interaction between FcRn and IgG without interfering with FcRn-albumin interactions. In this study, we evaluate the effect of UCB4470 on circulating aPL and aPL-induced thrombus formation.
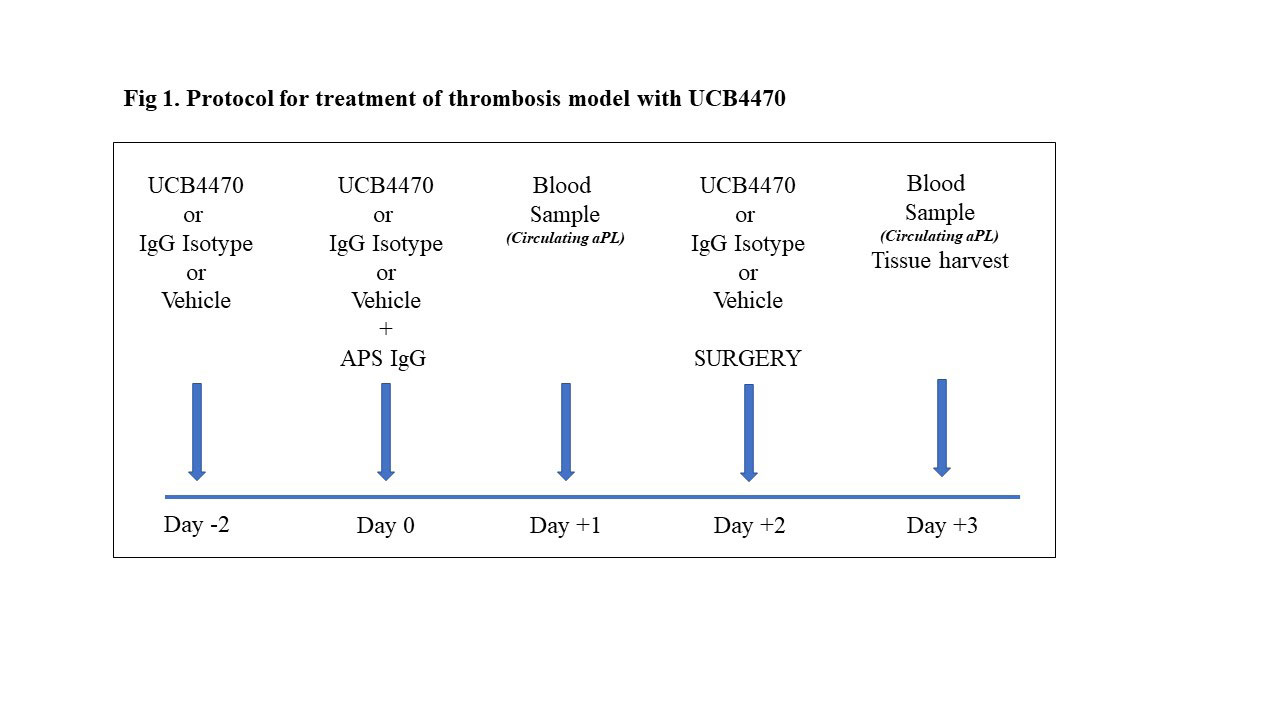
Methods: Balb/c mice were treated with either UCB4470 (30mg/kg), isotype control (30mg/kg) or vehicle by intravenous injection. This was repeated 2 days later, along with an intraperitoneal administration of human purified IgG (500mg/animal) pooled from 17 patients positive for IgG aCL and anti-b2GPI, and lupus anticoagulant (LA). Thrombus was induced 2 days after the aPL+UCB4470 or isotype or vehicle injection using the stenosis model in the inferior vena cava (IVC) in adult male BALB/C mice. A further dose of UCB4470, isotype control, or vehicle was administered immediately after thrombus induction. Thrombus and surrounding vein wall were excised 24hrs after surgery. Thrombi were separated from the IVC wall and weighted. Blood samples were obtained at day 1 and day 3 to measure total human IgG and IgG aCL and anti-b2GPI by commercial ELISA. Protocol is shown in figure 1.
Results: Thrombi from UCB4470-treated animals were lighter than those treated with aPL only (22±1mg (n=21) vs. 27±1mg (n=17), respectively; P=0.034). There were no differences in thrombus weight between UCB4470-treated animals and control animals (22±1mg vs. 21±2mg (n=12), respectively; P=0.6); or between animals treated with isotype control and aPL only (26±1mg (n=20) vs. 27±1mg, respectively; P=0.5).
UCB4470 treatment reduced total IgG levels between day 1 and day 3 (11.6±1.6mg/ml (n=26) vs. 3.7±0.5mg/ml (n=24), respectively, P< 0.0001), while there was no difference found for the isotype control (20.7±2.6mg/ml (n=24) vs. 20.2±2.7mg/ml (n=22), P=0.68), or the aPL only treated group (21.6±3.5mg/ml (n=24) vs. 18.9±2.1mg/ml (n=22), P=0.05) between these time points.
On day 3, titres of IgG aCL were significantly lower in the UCB4470-treated group when compared with the isotype control group (8.97±0.02GPL vs. 9.6±0.11GPL, P< 0.0001) and aPL only treated group (9.29±0.06GPL, P< 0.0001). Titres of IgG anti-b2GPI were significantly lower in the UCB4470-treated group when compared with the isotype control group (6.98±0.02GPL vs. 7.7±0.15GPL, P< 0.0001) and aPL only treated group (7.5±0.09GPL, P< 0.0001). A good correlation between IgG aCL and anti-b2GPI was seen in mouse blood (R2 0.8487, P< 0.0001)
Conclusion: Blocking FcRn ameliorates thrombus formation in a mouse model of aPL-induced thrombosis. Blocking FcRn also decreased the concentration of IgG aCL and anti-b2GPI in this model. These data indicate that therapeutic inhibition of FcRn may be beneficial in APS.
To cite this abstract in AMA style:
Bertolaccini M, Ioannou Y, Neale H, Saha P, Smith A. Evaluation of the Effect of UCB4470 (anti-mouse FcRn) on Amelioration of Thrombosis in a Mouse Model of Antiphospholipid (aPL)-induced Thrombosis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-effect-of-ucb4470-anti-mouse-fcrn-on-amelioration-of-thrombosis-in-a-mouse-model-of-antiphospholipid-apl-induced-thrombosis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-effect-of-ucb4470-anti-mouse-fcrn-on-amelioration-of-thrombosis-in-a-mouse-model-of-antiphospholipid-apl-induced-thrombosis/