Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Little is known about the long-term effects of pegloticase therapy or what urate-lowering therapy (ULT) patients subsequently receive when they discontinue pegloticase. This analysis examined alternative ULT use, serum urate (SU) changes, systemic inflammatory measures, and renal function following pegloticase discontinuation.
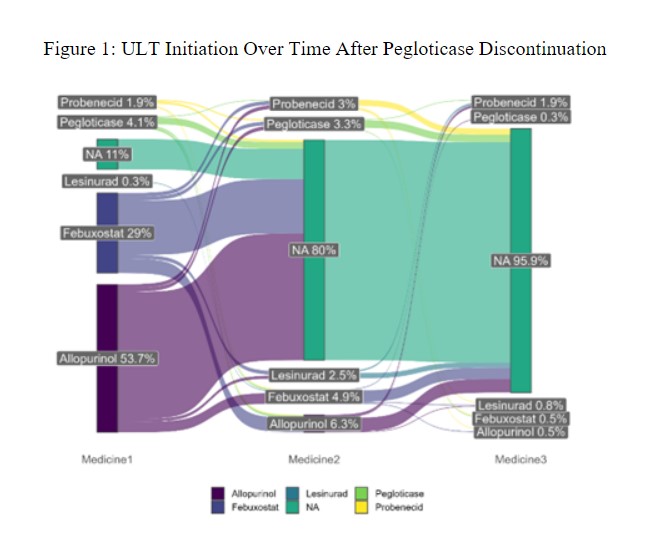
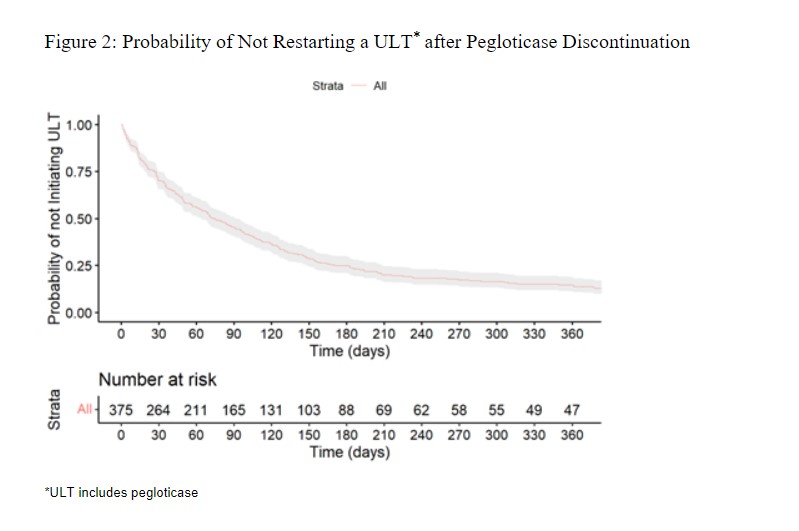
Methods: We conducted a retrospective analysis of gout patients discontinuing pegloticase using the ACR’s Rheumatology Informatics System for Effectiveness (RISE) registry from 01/2016 through 06/2022. We defined pegloticase discontinuation as a gap ≥12 weeks after a pegloticase infusion. We examined outcomes beginning two weeks after the last dose of pegloticase (defined as the ‘index date’), an interval selected based on its typical every other week dosing schedule. Changes in lab values (treatment effect) following pegloticase discontinuation were examined by comparing measurements obtained within 15 days of the second pegloticase dose and those obtained after pegloticase discontinuation. We analyzed changes in SU, eGFR, CRP, and ESR using descriptive statistics. A Sankey plot was used to describe ULT medication use after the index date, and Kaplan Meier curves assessed the probability of starting post-pegloticase ULT over time.
Results: We identified 375 patients with gout who discontinued pegloticase and had paired labs. We observed lab changes in patients who discontinued pegloticase with median (IQR) differences of SU: 2.4 mg/dL (0.0, 6.3); eGFR: -1.9 mL/min (-8.7, 3.7); CRP: -0.8 mg/L (-12.8, 0.0); and ESR: -4.0 mm/hr (-13.0, 0.0), compared to pre-discontinuation values.
Of those who discontinued pegloticase, 83% started other ULTs (Figure 1), and 8% restarted pegloticase. Of those that started oral ULTs, 63% began allopurinol and 34% began febuxostat. The time to starting (or restarting) ULT is shown in Figure 2. With SU measured at least >=30 days following ULT start, 53% of patients had an SU ≤6 mg/dL with a median SU of 5.8 mg/dL (IQR: 3.6, 8.4).
After starting a new ULT, post-ULT median (IQR) SU values were 5.8 (4.7, 7.0) and,5.8 (4.5, 7.9) mg/dL for users of allopurinol and febuxostat, respectively. In contrast, patients who restarted pegloticase achieved a median SU of 0.9 mg/dL (IQR: 0.2, 9.7) after a median of 156 days (IQR: 131.0, 609.0) since prior discontinuation.
Conclusion: Pegloticase treats uncontrolled gout in patients who fail to respond to xanthine oxidase inhibitors, but the development of anti-drug antibodies, and subsequent loss of urate-lowering response may require pegloticase discontinuation. An important minority of patients fail to receive any ULT after cessation of pegloticase. Additionally, many patients who were able to restart restarting pegloticase after a prolonged gap in therapy achieved had its expected resulting SU-lowering effect with therapy even after a gap in therapy, although the context for the interruption needs to be further explored. Future studies should focus on the optimal management of gout patients who discontinue or have meaningful gaps in pegloticase treatment.
To cite this abstract in AMA style:
Holladay E, Mudano A, Xie F, Zhang J, Mikuls T, LaMoreaux B, Padnick-Silver L, Curtis J. Evaluation of Outcomes Following Discontinuation of Pegloticase Therapy [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-outcomes-following-discontinuation-of-pegloticase-therapy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-outcomes-following-discontinuation-of-pegloticase-therapy/