Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: A double-blind, randomized, placebo-controlled trial (TRIUMPH; NCT02558439) with a single 1 mg intra-articular (IA) injection of CNTX-4975 (highly purified trans-capsaicin) into one knee for management of moderate to severe pain associated with knee osteoarthritis (OA) revealed a statistically significant and clinically meaningful decrease in pain with walking vs placebo through 24 weeks post-dose. The present open-label study (NCT03472677) evaluated the efficacy and safety of a prefilled syringe with an aqueous formulation of CNTX-4975. Presented here are the 6-week outcomes after bilateral CNTX-4975 injections.
Methods: Subjects (aged 45-75 y) with bilateral moderate to severe painful knee OA (numeric pain rating scale [NPRS, 0–10]) score of 4–9 inclusive required for enrollment) for ≥3 months before study entry received a single IA injection of CNTX-4975 1 mg into each knee, with 7 days (±2 days) between injections. ACR criteria were met by 67% of subjects for knee OA as assessed by knee pain, radiographic findings, and age >50 y; the other 33% had pain and radiographic findings, but were aged 47–49 y. IA lidocaine 2% (without epinephrine, 15 mL) was given 3–30 minutes before injection of CNTX-4975. Skin around the knee was cooled before lidocaine injection and throughout the procedure using different methods, and temperature changes in the knee joint were documented with an IA temperature probe. Pain with walking over the previous 24 hours was rated by the NPRS at baseline and at 6 weeks (trial end). Subjects also rated the change in pain in each knee using the Patient Global Impression of Change (PGIC) scale (7-point categorical scale from very much worse to very much improved). Knees were examined to determine the presence of injection site reactions, and adverse events were recorded.
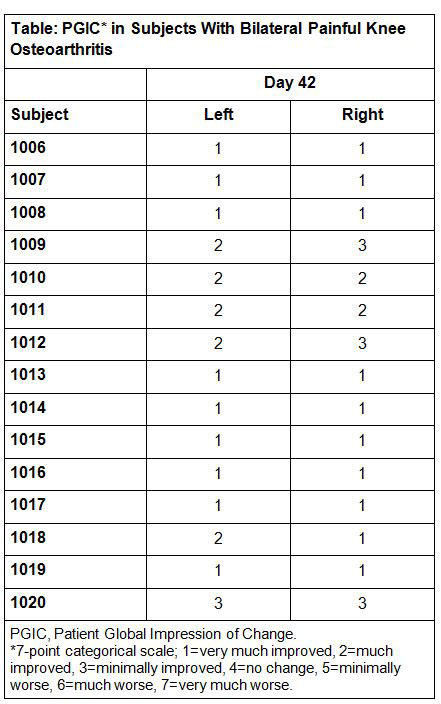
Results: Fifteen subjects (30 knees) with bilateral knee OA (12 male, 3 female; mean age, 57 y) were enrolled and completed the study. Baseline and day 42 NPRS scores were averaged across both knees. At 6 weeks, pain rating by NPRS was reported as 0 in 13 of 30 assessments (15 subjects, both knees), and pain score was reported as ≤3 of 10 for 26 of 30 knees. A similar pattern of improvement was evident with the PGIC (Table): in 26 of 30 recordings, subjects described pain at trial end as much or very much improved. No subject reported pain as being unchanged or worse in either knee. Treatment-emergent adverse events (TEAEs) reported on the day of injection were nausea (n=3), headache (n=2), vasovagal attack, dizziness, elevated blood pressure, and tachycardia (n=1 each); all were reported as mild with 2 (vasovagal attack and 1 report of nausea) deemed possibly related to treatment. TEAEs reported after the day of injection were insect bite (n=2) and hay fever, vasovagal attack, back pain, acid reflux (esophageal), allergic conjunctivitis, and bruise (n=1 each); all were mild and deemed unlikely or not related to study treatment.
Conclusion: Pain in bilateral osteoarthritic knees was rated much or very much improved in 87% of assessments, and >40% of knees were reported pain free at 5–6 weeks post CNTX-4975 injection.
To cite this abstract in AMA style:
Stevens R, Guedes K, Mistry N, Tiseo P, Lascelles D, Mendoza M, Ball D. Evaluation of Intra-articular CNTX-4975 in Subjects with Painful Bilateral Knee Osteoarthritis: Effects on Pain with Walking and Patient Impression of Change in Pain [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-intra-articular-cntx-4975-in-subjects-with-painful-bilateral-knee-osteoarthritis-effects-on-pain-with-walking-and-patient-impression-of-change-in-pain/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-intra-articular-cntx-4975-in-subjects-with-painful-bilateral-knee-osteoarthritis-effects-on-pain-with-walking-and-patient-impression-of-change-in-pain/