Session Information
Date: Monday, November 8, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster III: Patient Preferences (1153–1169)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Despite being used for more than 70 years as a conventional (cs) DMARD, very little is known about the overall side effect (SE) profile of hydroxychloroquine (HCQ). In 2020 HCQ became well-known due to the COVID 19 pandemic. We set out to understand the patient-reported side effect of HCQ use in adults with RA, SLE, and other RMDs (excluding Osteoarthritis and Fibromyalgia).
Methods: Data provided by participants in the Forward Databank observational registry from 1999 through 2020. HCQ use was measured at enrollment and every 6 months with follow up questionnaires. Incident HCQ users were defined as new users (no HCQ use documented in a prior questionnaire) or having a 6-month maximum HCQ duration prior to FORWARD entry. Prevalent HCQ users were any user irrespective of duration of HCQ treatment. These questionnaires asked about all SEs to medications, including severity of side effect, certainty of medication as cause of side effect, and affected body systems. We analyzed incident rates of side effects overall and by HCQ categorical use: monotherapy, HCQ with concomitant use of another csDMARD, or HCQ with concomitant use of a biologic (b)DMARD or targeted synthetic (ts) DMARD.
Results: Overall, 5,685 patients were included in the initiator group and 13,018 patients were included in the prevalent group, with the majority of patients taking HCQ for RA and SLE. In both the initiator and prevalent cohorts, the majority of patients with RA were on HCQ and another DMARD. In the other RMD and SLE groups, the majority of patients were on HCQ monotherapy. Eighty four percent of patients taking HCQ for any cause did not experience any side effects in both initiator and prevalent groups. Sixteen percent of all RA patients, 16.5% of SLE and 18% of other RMD patients noted a side effect from HCQ in the initiator group, vs 16.2% of RA, 18.8% of SLE and 17.5% of other RMD noted in the prevalent group. Of those patients, 55% in the RA, 41% in the SLE and 39.3% in the other RMD groups discontinued the medication in the initiator group, with very similar results noted in the prevalent group. Hospitalization secondary to a hydroxychloroquine side effect was observed in 1% of RA patients, 2.5% of SLE patients and no patients with other RMDs in the initiator group vs 1% of RA, 2.8% of SLE and no patients with other RMD’s noted in the prevalent group. In both the prevalent and initiator groups, the predominant last reported side effects in annualized incidence rates per 1000 patient-years were gastrointestinal (GI; e.g., nausea, diarrhea, 12.35 prevalent, 12.84 initiator), integumentary (e.g., rash, itching, 7.61, 8.6), and ocular (e.g., visual disturbance, 8.54, 7.42), respectively over all 3 disease groups.
Conclusion: This is the largest study of its kind conducted that reviews patient reported side effects over a 20-year period. Our findings confirm the overall low incidence of reported serious side effects from short and long- term hydroxychloroquine use for the treatment of SLE, RA and other RMDs.
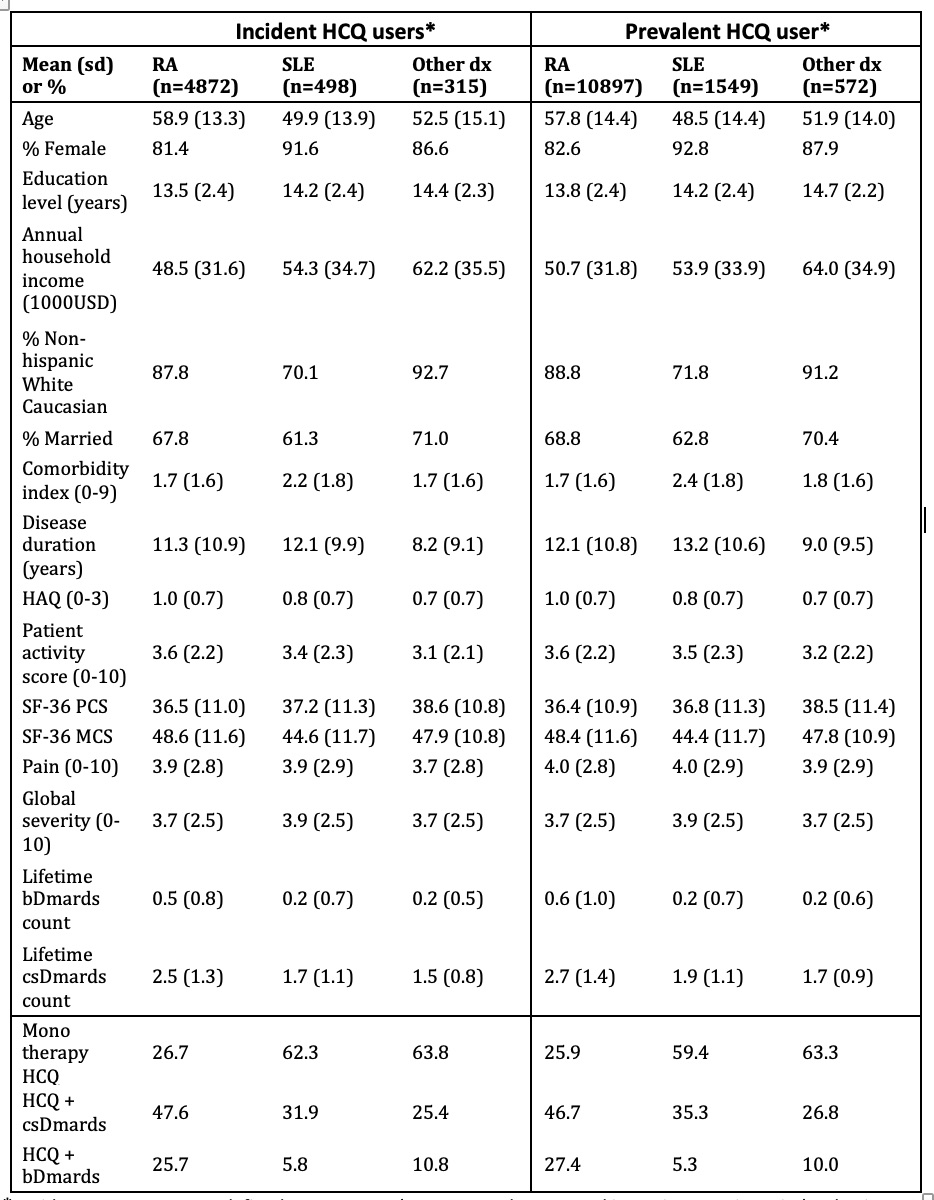 Table 1. Baseline characteristics of prevalent and incident HCQ users by diagnoses. * Incident HCQ users were defined as new users (no HCQ use documented in a prior questionnaire) or having a 6-month maximum HCQ duration prior to FORWARD entry. Prevalent HCQ users were any user irrespective of duration of HCQ treatment. Baseline is defined as the phase preceding HCQ initiation or the first observation in the study if duration HCQ≤6 months. For prevalent users the definition is the latter.
Table 1. Baseline characteristics of prevalent and incident HCQ users by diagnoses. * Incident HCQ users were defined as new users (no HCQ use documented in a prior questionnaire) or having a 6-month maximum HCQ duration prior to FORWARD entry. Prevalent HCQ users were any user irrespective of duration of HCQ treatment. Baseline is defined as the phase preceding HCQ initiation or the first observation in the study if duration HCQ≤6 months. For prevalent users the definition is the latter.
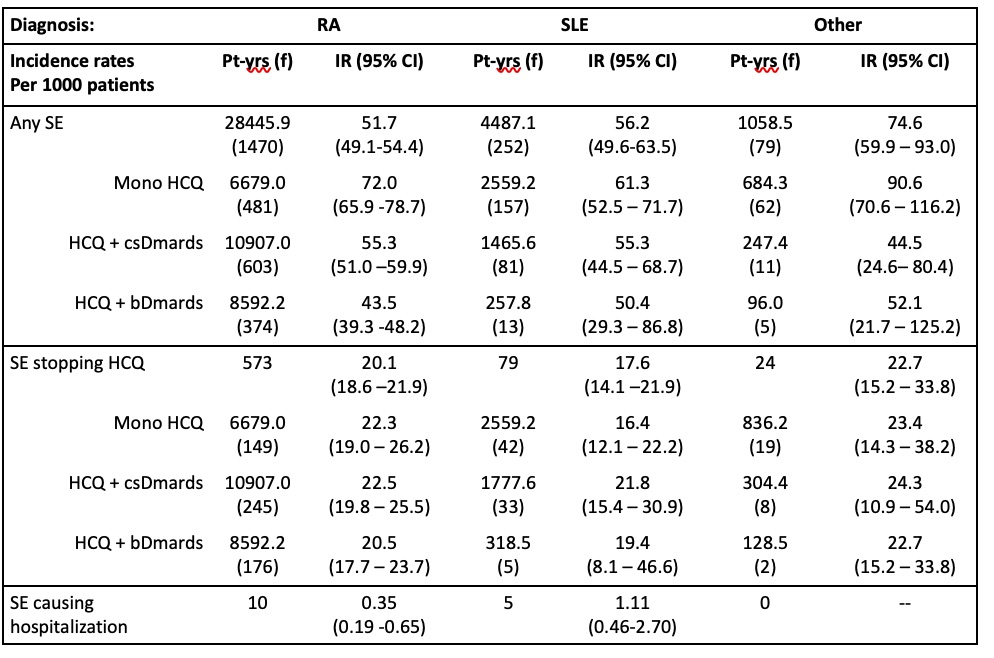 Table 2. HCQ use and experience of side effects due to HCQ of the last side effect (multiple infections)
Table 2. HCQ use and experience of side effects due to HCQ of the last side effect (multiple infections)
To cite this abstract in AMA style:
Meyler D, Pedro S, Erickson A, Katz P, Michaud K. Evaluation of HCQ Side Effects in New and Prevalent Users over a 20 Year Period Using a Large Database [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-hcq-side-effects-in-new-and-prevalent-users-over-a-20-year-period-using-a-large-database/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-hcq-side-effects-in-new-and-prevalent-users-over-a-20-year-period-using-a-large-database/
