Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA.1,2 Using two mass spectrometry (MS)-based proteomic approaches (targeted multiple reaction monitoring [MRM] and unbiased discovery) for analysis of baseline (BL) serum samples from patients (pts) with PsA receiving tofacitinib, adalimumab (ADA), or placebo (PBO), 181 candidate biomarker peptides were identified that may predict treatment response in PsA.3 We evaluated an updated, quantitative MRM assay that incorporated the differentially expressed peptides previously found and assessed its ability to predict treatment response.
Methods: Pts with PsA receiving tofacitinib 5 or 10 mg twice daily, ADA, or PBO in OPAL Broaden (NCT01877668)1 were classed as responders (R) or non-R (NR) by Month 3 PsA Disease Activity Score (R: lowest score ≤ 3.2; NR: highest score > 3.2). BL serum samples were analyzed via an updated targeted MS MRM assay, including peptides representing the proteins previously identified as differentially expressed in R vs NR serum samples using MRM analysis of an in-house panel (‘PAPRICA’) and unbiased discovery liquid chromatography-tandem MS (LC‑MS/MS). Peptide data quality was examined manually; low signal:noise ratio (< 6) and/or poor peak shape identified those not meeting quality assessment. PAPRICA data were normalized to (1) stable isotopically labeled (SIL) peptide spike-ins and (2) an endogenous peptide panel representing total serum protein abundance (TSPA). Univariate analyses (Student’s t-test with no multiplicity adjustment) and multivariate machine learning Random Forest (RF) modeling4 were performed.
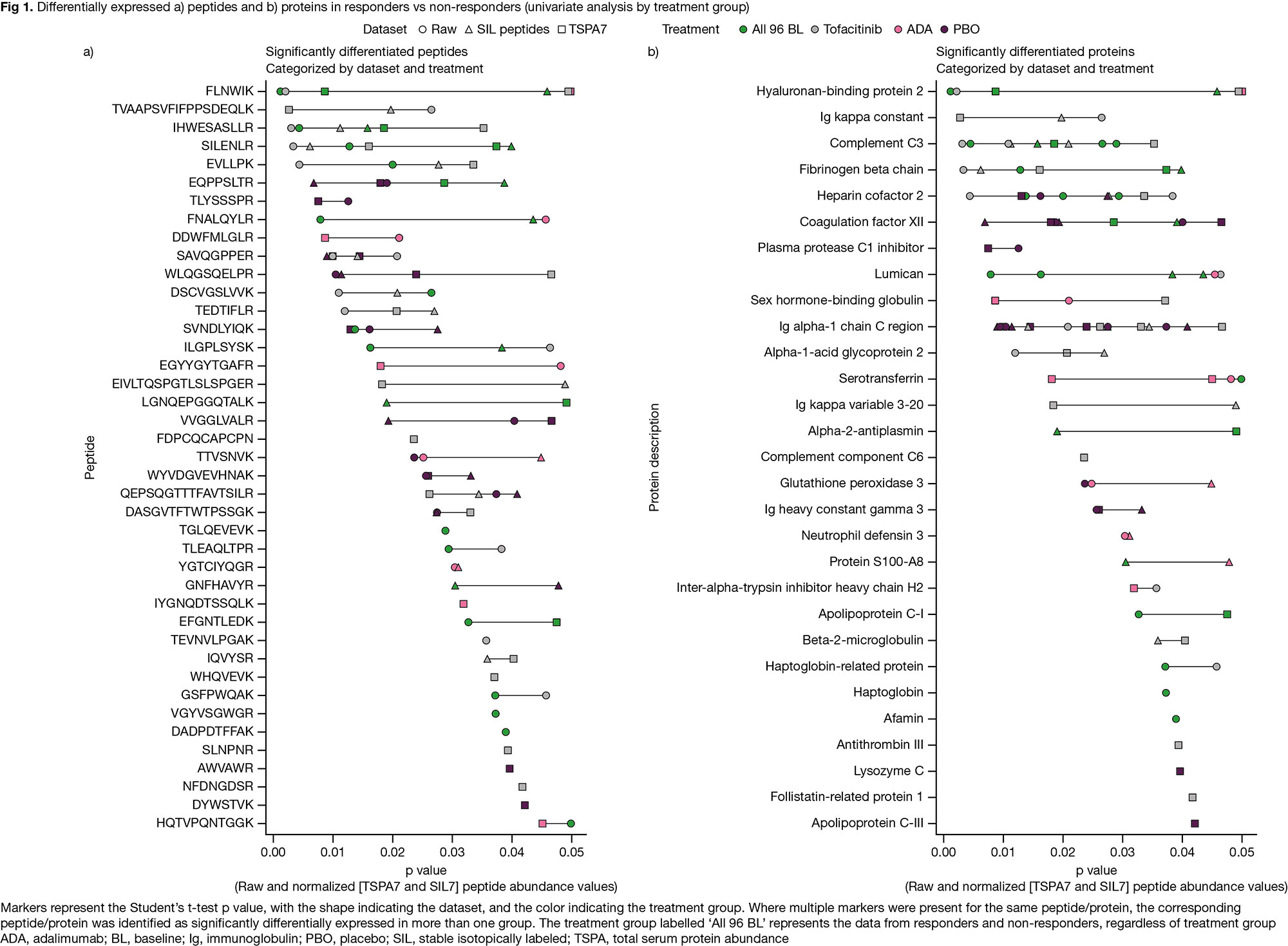
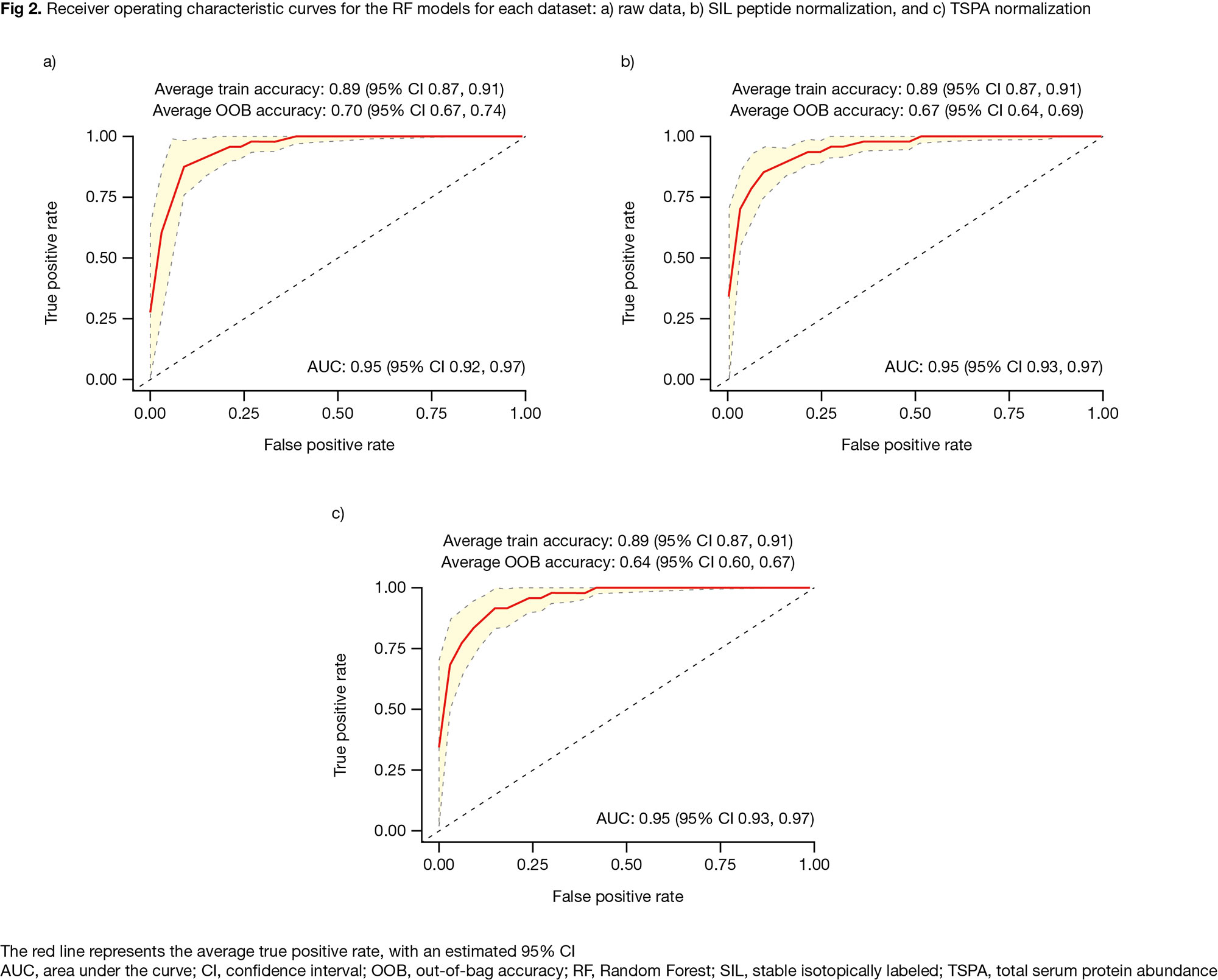
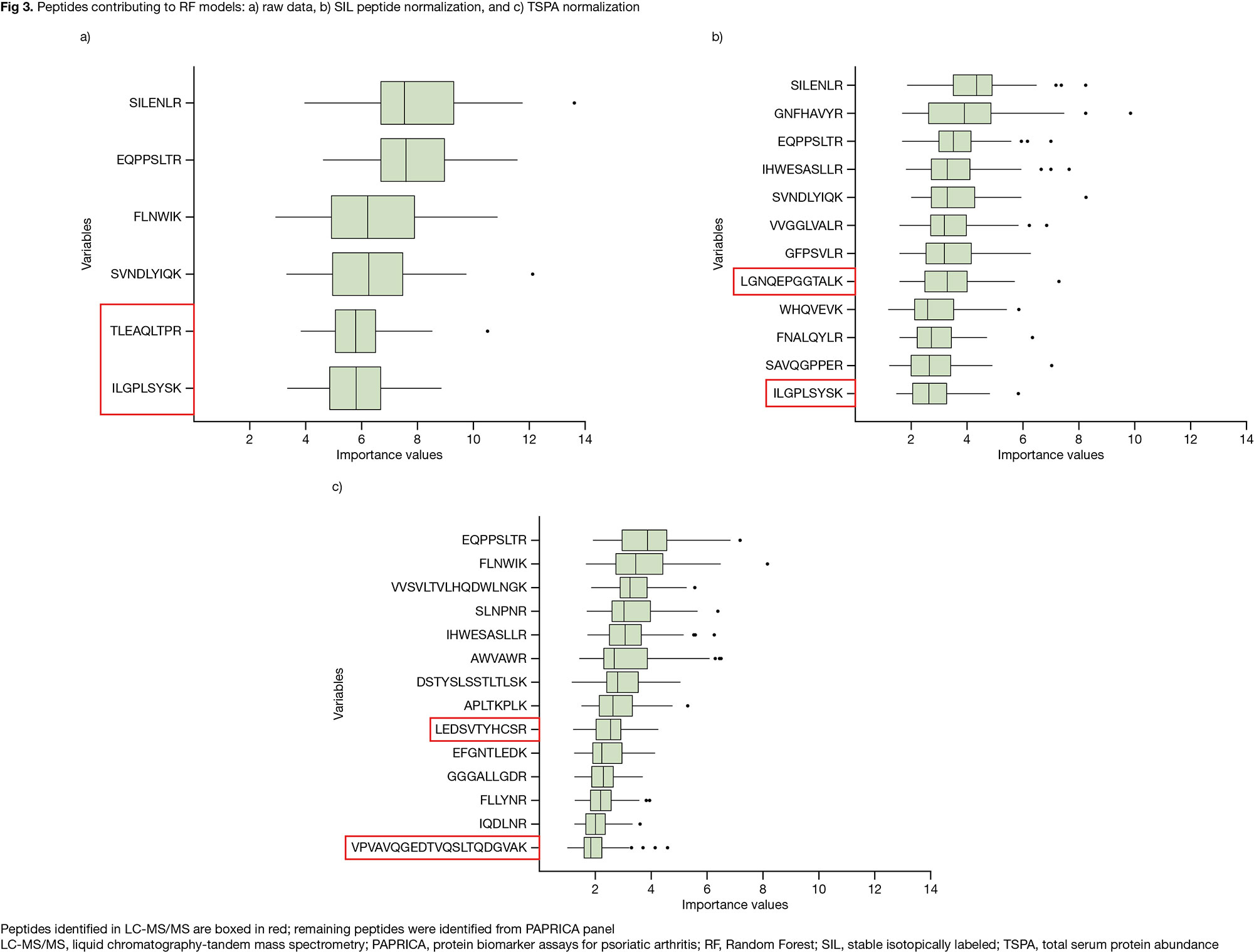
Results: In quality control, 40 peptides were removed due to low signal:noise ratio and/or poor peak shape. Univariate analysis identified 41 peptides (34 from PAPRICA; 7 from LC-MS/MS) differentially expressed (p £ 0.05) in R (N=47) vs NR (N=49). In all, 21/9/13 peptides were differentially expressed in the tofacitinib/ADA/PBO groups (Fig 1a). The 41 peptides mapped to 29 unique proteins (16/8/8 differentially expressed in the tofacitinib/ADA/PBO groups; Fig 1b). RF analysis generated models with a modest ability to predict treatment response; performance was broadly equivalent to PAPRICA3 (Fig 2). These models, made up of 6/12/14 variables from the raw/SIL normalized/TSPA normalized datasets, mostly contained peptides originally identified in PAPRICA; 2 peptides per model were identified in LC-MS/MS (Fig 3).
Conclusion: Analysis of an updated panel of candidate biomarkers for predicting treatment response in pts with PsA identified 41 differentially expressed peptides from 29 proteins associated with R vs NR to tofacitinib, ADA, or PBO. RF models had a modest ability to predict BL treatment response. Further evaluation is ongoing to verify these candidate predictors of response, and we will report how these proteins map to biologic processes, pathways, and networks.
1. Mease et al. N Engl J Med 2017; 377: 1537-50
2. Gladman et al. N Engl J Med 2017; 377: 1525-36
3. Waddington et al. Ann Rheum Dis 2022; 81 (S1): 840
4. Breiman. Mach Learn 2001; 45: 5-32
Study sponsored by Pfizer. Medical writing support was provided by J Bardos, CMC Connect, and funded by Pfizer.
To cite this abstract in AMA style:
Waddington J, Zhou R, Coleman O, Wundervald B, Parnell A, Chandran V, Fallon L, Chapman D, Pollock R, Deng S, FitzGerald O, Pennington S, Mease P. Evaluation of Candidate Protein Biomarkers to Predict Treatment Response in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-candidate-protein-biomarkers-to-predict-treatment-response-in-patients-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-candidate-protein-biomarkers-to-predict-treatment-response-in-patients-with-psoriatic-arthritis/