Session Information
Date: Monday, November 9, 2020
Title: Pediatric Rheumatology – Clinical Poster III: SLE, Vasculitis, & JDM
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Rituximab is standard therapy for autoimmune brain disease (ABD) including autoimmune encephalitis (AE) and Neuromyelitis Optica Spectrum Disease (NMOSD). Recommendations for continuing concurrent intravenous immunoglobulin (IVIG) during rituximab treatment in patients with ABD vary. Mechanisms of rituximab B cell depletion include antibody-dependent cell-mediated and complement mediated cytotoxicity. In contrast, IVIG’s immunomodulation includes attenuation of many of these same mechanisms. Despite the frequent use of IVIG as adjunct therapy, there is little data on how concomitant use may affect B-cell depletion and efficacy.
The objective of this study was to determine if concurrent treatment of rituximab with IVIG altered B-cell depletion and time to repopulation in pediatric patients with ABD. We also assessed rates of hypogammaglobinemia (hypogam), duration of continuous maintenance therapy, and reasons for discontinuation of therapy.
Methods: We conducted a retrospective chart review of 58 patients who received rituximab for ABD. All patients met criteria for probable autoimmune encephalitis defined by a multidisciplinary team including a pediatric neurologist, rheumatologist and psychiatrist or NMOSD per established criteria. 49 patients met our inclusion criteria: follow-up for >1 year, standard rituximab induction, and CD19/CD20 counts recorded post-treatment.
We calculated time from most recent rituximab dosing to CD19/CD20 repopulation (defined as CD19 or CD20 >0.2%) and noted if IVIG was given within 4 weeks. We evaluated duration of rituximab therapy, concurrent IVIG, B cell repopulation, frequency of hypogam and serious infections. We noted the longest consecutive period of maintenance therapy for each patient. Dosing intervals for maintenance therapy varied from every 3 to 12 months, reflecting evolving recommendations for optimal dosing and early repopulation. Rationale for discontinuation was determined through chart review.
Results: 161 doses of rituximab were given in 49 patients, with 125 given concurrently with IVIG and 36 without. There was no statistical difference in B-cell repopulation in concomitant treatment compared to rituximab alone, in mean time to repopulation, or percent requiring escalation to next line therapy, as noted in Table 1.
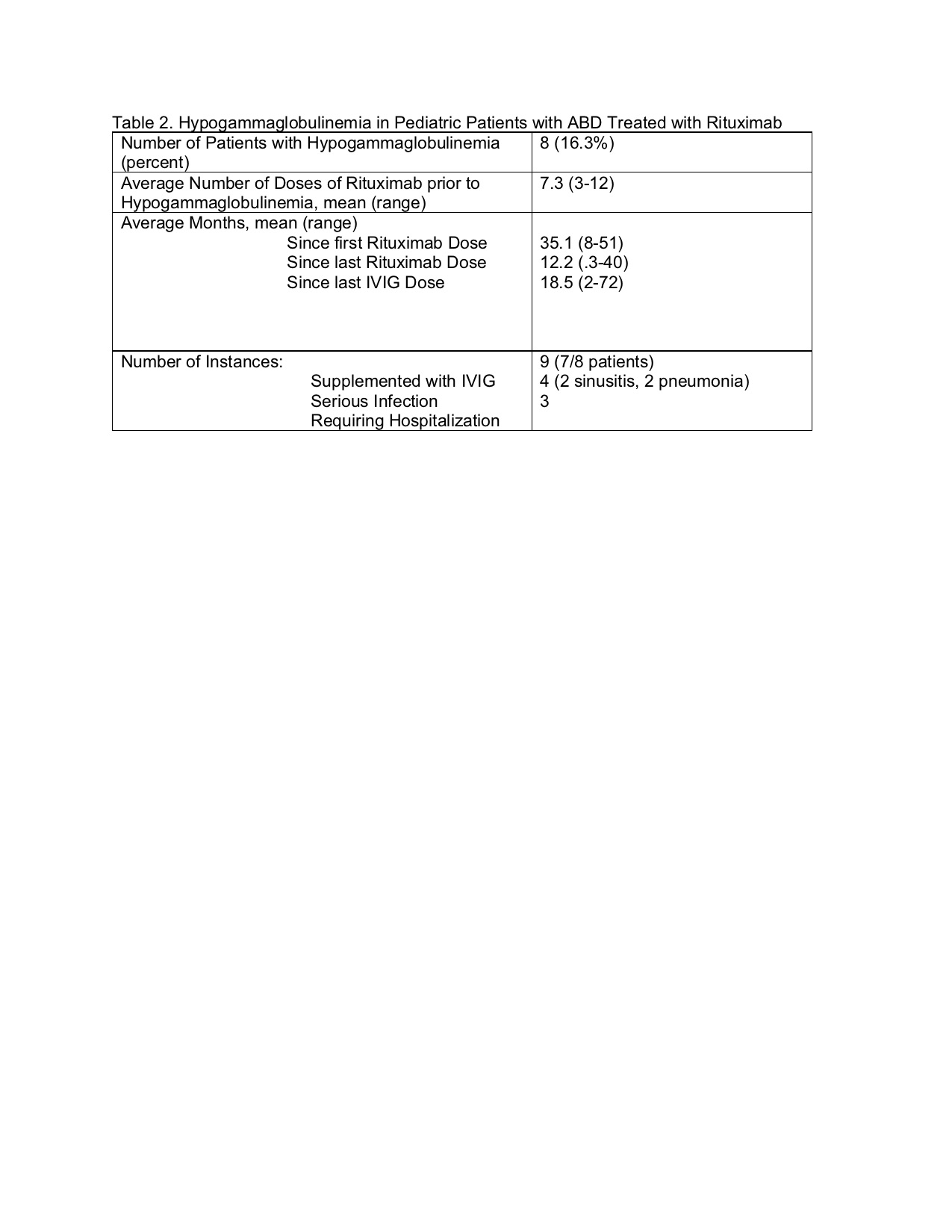
We observed hypogam in only 8 of our patients (16% of study population). All patients with serious infection had prompt recovery with replacement dose IVIG and antibiotics. Hypogam was noted on average 12.2 months after last rituximab and 18.5 months after last IVIG.
33 patients (67%) received prolonged treatment ( >1 year), of which 14 were on maintenance therapy 13-24 months and 19 on therapy for over 24 months. Of note, NMO patients in our practice continue rituximab chronically given high rates of relapse, even in clinical remission. 35% of patients continued rituximab at the completion of this study, 29% ended therapy for remission, 16% escalated to a different DMARD and 12% failed to respond with no escalation.
Conclusion: There was no difference in rates or time to B cell repletion in patients treated with rituximab alone vs concurrent IVIG treatment. Rates of hypogammaglobinemia were low in our cohort.
To cite this abstract in AMA style:
Wilsey A, Cannon L, Johannes S, Van Mater H. Evaluation of B-cell Depletion with Rituximab and IVIG Concurrent Treatment in Pediatric Autoimmune Brain Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-b-cell-depletion-with-rituximab-and-ivig-concurrent-treatment-in-pediatric-autoimmune-brain-disease-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-b-cell-depletion-with-rituximab-and-ivig-concurrent-treatment-in-pediatric-autoimmune-brain-disease-2/