Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Reducing the dosage of biologic drugs in patients with rheumatoid arthritis (RA) who have achieved remission is a viable and necessary strategy. To identify RA patients who would benefit most from this approach, flare prediction models play an essential role. The aim of this project was to study whether biological therapy reduction is associated with joint flares and to develop a predictive model for joint flare-ups.
Methods:
Patients were selected from the OPTIBIO protocol (Eudra-CT code 2012-004482-40). This multicenter, open-label, randomized, controlled, phase IV trial included patients over 18 with rheumatoid arthritis (RA) who met the 1987 ACR criteria, were in remission for at least 6 months, and on biologic therapy. Clinical-demographic variables, disease activity indices, and serological markers were collected. Flares were determined over a 36-month follow-up. Sociodemographic, clinical, and genetic variables (including 15 SNPs and 16 serum proteins) were analyzed for patients with flare-ups and those in remission to identify risk factors. Flare-free survival was estimated using the Kaplan-Meier method and compared with the log-rank test. Cox models were developed to predict flares. Two models were evaluated: (i) clinical and (ii) clinical + molecular. Predictive accuracy was assessed by global and time-dependent AUC at 12, 18, and 24 months.
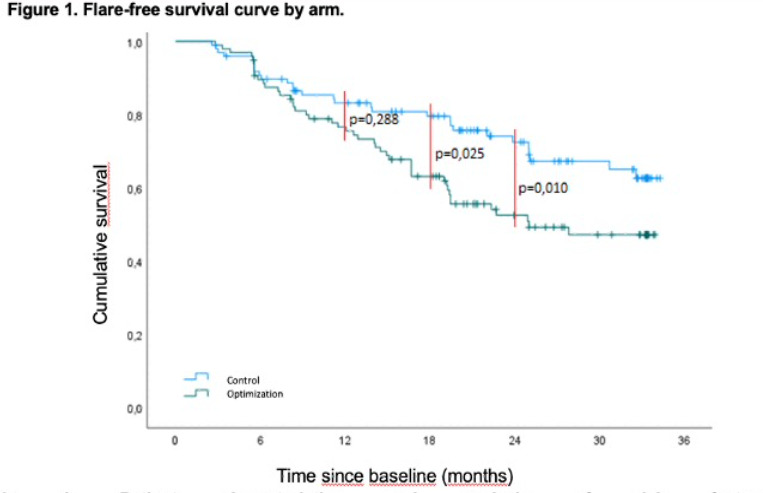
Results: A total of 196 patients were included: 99 in the control group and 96 in the optimization group. There were no statistically significant differences between the groups. By the end of follow-up, 75 flares were recorded: 30 (40%) in the control group and 45 (60%) in the optimization group (p=0.02). Flares at 12, 24, and 36 months were: 16 (53.3%), 12 (40%), and 2 (7%) in the control group, and 24 (53.3%), 20 (44.4%), and 1 (2%) in the optimization group. The flare-free survival curve showed a significant difference between groups from month 18 (p=0.025). Survival probability at 18 months was 78.7% versus 63.1% and 71.7% versus 52.5% at 24 months in the control and optimization groups, respectively (Figure 1).
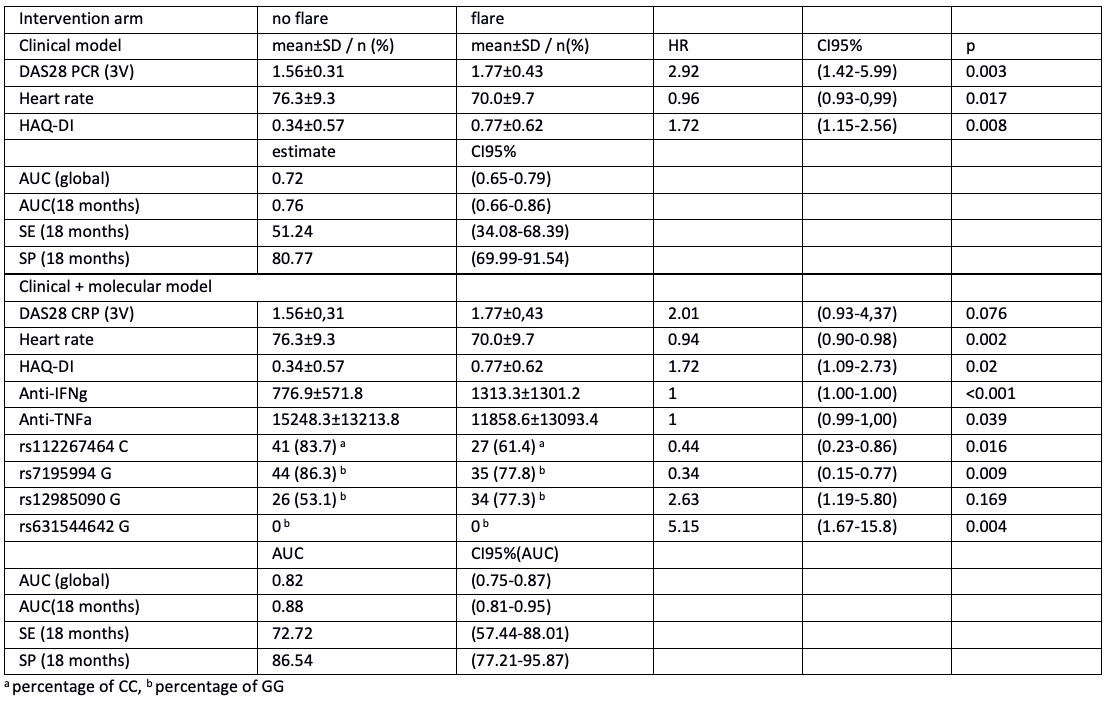
Clinical and sociodemographic variables associated with flares in the optimization group were heart rate, CRP, VAS pain, VAS physician, DAS28-ESR 3v, DAS28-CRP 3v, SDAI, and HAQ-DI. The best predictive clinical model included DAS28-CRP 3v, HAQ-DI, and HR. This model showed a global AUC = 0.72 and time-dependent AUC ranging from 0.76 to 0.84 at 18 and 24 months, respectively. The clinical plus genetic and proteomic variables model showed an increase in time-dependent AUC from 0.88 at 18 months to 0.90 at 24 months, with a global AUC = 0.81 (Table 1).
Conclusion: The biologic dose reduction strategy in patients with RA in remission used in this study was associated with an increased risk of joint flares. We have developed an instrument to predict flare-ups, which is currently being validated in a clinical trial.
To cite this abstract in AMA style:
Galindo Domínguez L, Acasuso B, Balboa-Barreiro V, Fernández-Tajes J, Cañete J, Fernández-Gutiérrez B, González-Álvaro I, Pablos J, Bejerano-Herreria C, Silva-Díaz M, De-Toro-Santos F, Oreiro N, Blanco f. Evaluating Dose Reduction of Biologic Treatments in Rheumatoid Arthritis: Predicting Flares Using Clinical and Molecular Biomarkers [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluating-dose-reduction-of-biologic-treatments-in-rheumatoid-arthritis-predicting-flares-using-clinical-and-molecular-biomarkers/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-dose-reduction-of-biologic-treatments-in-rheumatoid-arthritis-predicting-flares-using-clinical-and-molecular-biomarkers/