Session Information
Date: Monday, November 6, 2017
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The primary endpoint in SLE trials is usually response to therapy at a landmark visit. However, during a trial, patients may alternate between response and non-response states. Duration of response would therefore be important to assess, but the optimal approach for estimating response duration has not been determined. Analyzing response duration only among responders at a landmark visit can result in selection bias. Drop-outs and missed visits further complicate estimation of response duration. We addressed these issues with a multi-state Markov (MSM) model that was fit to quantify response duration and assess baseline predictors of transitions into and out of response in SLE patients receiving standard of care (SoC).
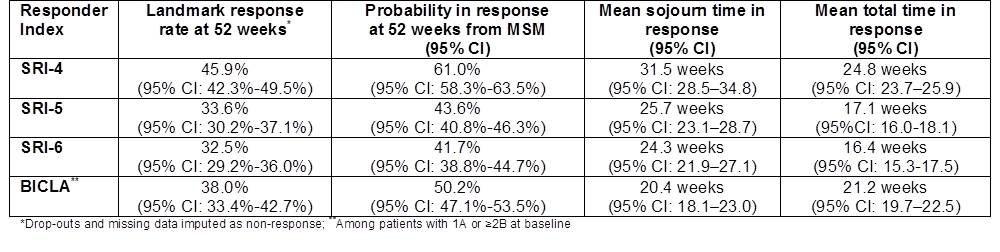
Methods: Data on 759 SLE patients with active disease (SLEDAI ≥ 6 at entry) randomized to SoC in 52 week trials was obtained from the Collective Data Analysis Initiative (CDAI) database of the Lupus Foundation of America. The following monthly response endpoints (without medication stipulations) were analyzed: SRI-4, SRI-5, SRI-6, and BICLA. A MSM model allowing for bi-directional transitions between response and non-response states was fit to estimate the probability of being in response at 52 weeks, average duration of response (sojourn time) and mean total time in response. Predictors of attainment and loss of SRI-5 response were also identified.
Results: Based on the MSM model, the probability of being in response at 52 weeks ranged from 42% (SRI-6) to 61% (SRI-4), higher than conventional 52 week landmark response rates that assume non-response for missing data. The estimated mean duration of response ranged from 20.4 weeks (BICLA) to 31.5 weeks (SRI-4). Mean total time in response over 52 weeks based on all patients was 16.4 – 24.8 weeks. After adjusting for baseline SLEDAI score, patients with lower anti-dsDNA titers were more likely to achieve and maintain SRI-5 response (p <0.001). Younger age (p < 0.001) and higher protein/creatinine ratio (p<0.001) were associated with higher frequency of SRI-5 response but also shorter response duration. Response duration was also shorter in patients who were non-White (p<0.001), had longer history of disease (p=0.03), and lower lymphocyte count (p=0. 001) at baseline.
Conclusion: Factors associated with greater disease severity were consistently associated with shorter response duration on SoC, despite exhibiting variable effects on the probability of achieving response at a given time. Response duration might therefore provide a more discriminating measure to distinguish effective investigational treatments from background SoC, although this remains to be tested. Multi-state models make better use of complex longitudinal clinical trial data and provide a more comprehensive view of the response profile and the role of patient characteristics in different aspects of response.
To cite this abstract in AMA style:
Kim M, Merrill JT, Kalunian KC, Hanrahan L, Izmirly PM. Estimating Duration of Response in Systemic Lupus Erythematosus (SLE) Trials [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/estimating-duration-of-response-in-systemic-lupus-erythematosus-sle-trials/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/estimating-duration-of-response-in-systemic-lupus-erythematosus-sle-trials/