Session Information
Date: Saturday, November 6, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster I (0387–0413)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
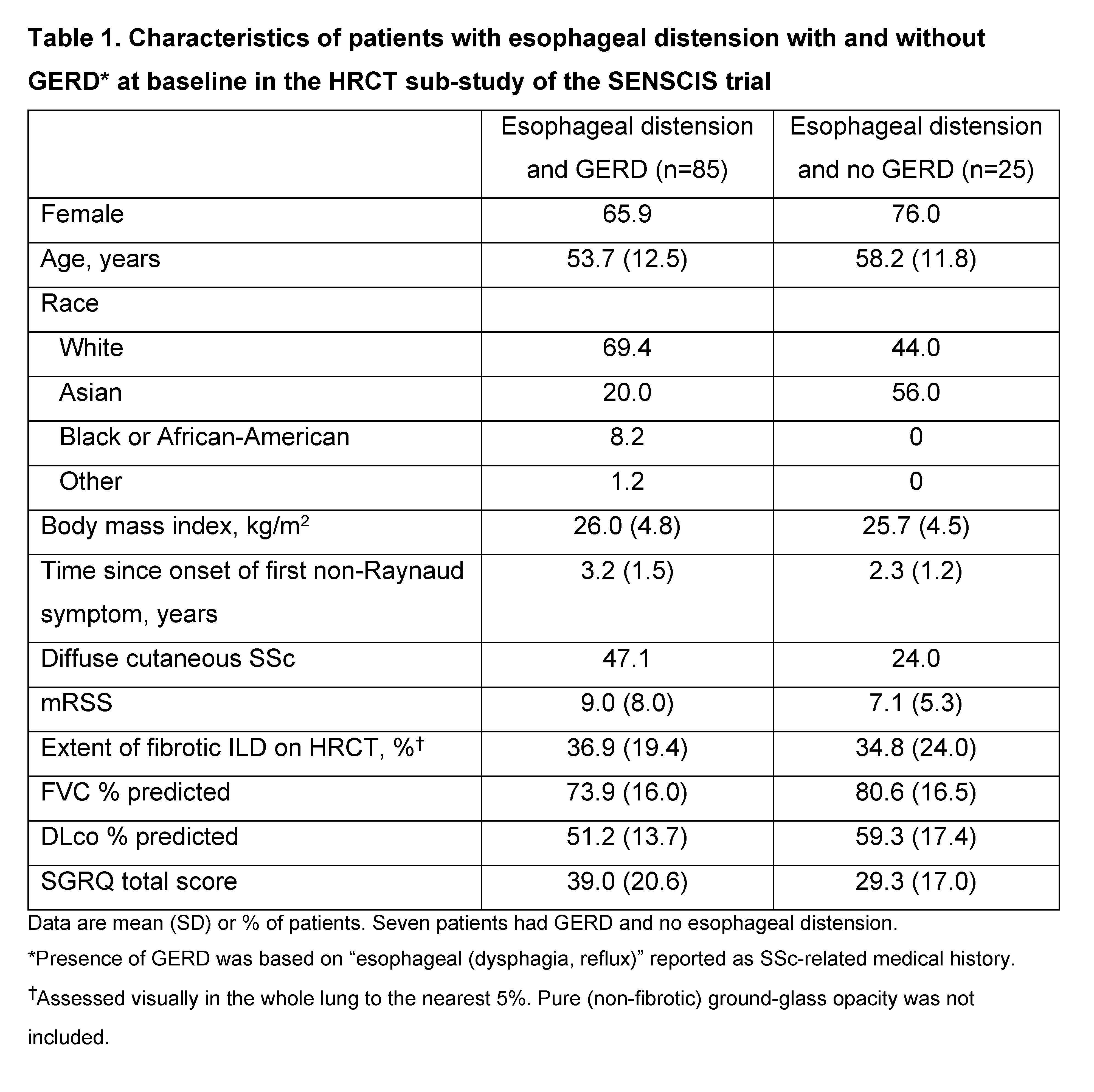
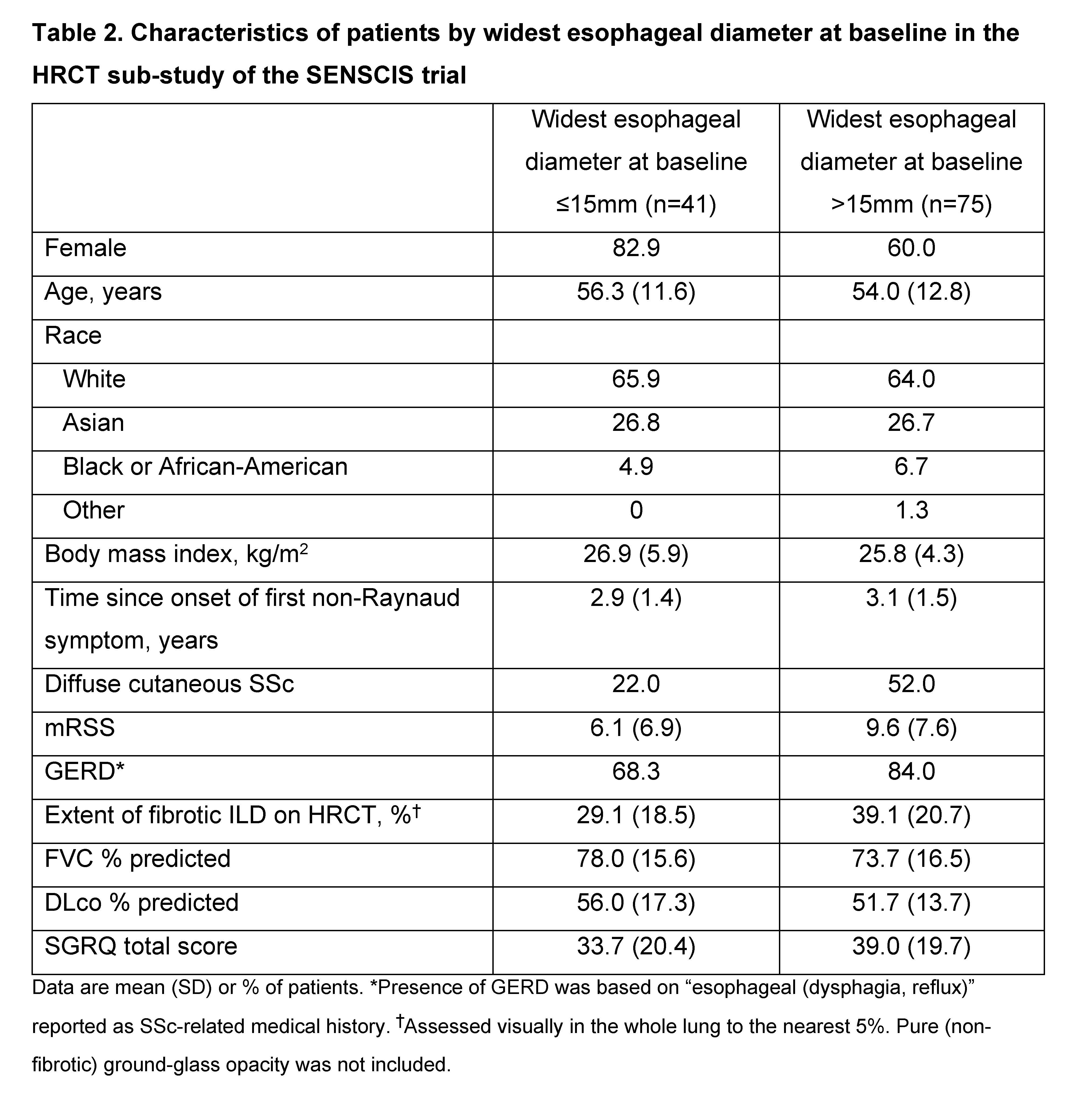
Background/Purpose: Upper gastrointestinal involvement such as esophageal dilation is frequently observed in patients with SSc-ILD and may be associated with the presence or severity of SSc-ILD. We assessed the characteristics of patients with SSc-ILD based on the presence of esophageal distension and gastroesophageal reflux disease (GERD), and based on widest esophageal diameter, using data from the SENSCIS trial.
Methods: Patients in the SENSCIS trial had SSc with first non-Raynaud symptom ≤7 years before screening and an extent of fibrotic ILD on HRCT (based on visual assessment of the whole lung) ≥10%. In a sub-study, the presence of esophageal distension and the widest esophageal diameter (i.e. the largest distance between internal esophageal mucosal limits for three diameter measurements) were assessed at baseline using high-resolution computed tomography (HRCT). In descriptive analyses, we assessed the characteristics of patients in subgroups by the presence (yes/no) of esophageal distension and GERD, and by widest esophageal diameter ≤15mm vs >15mm at baseline.
Results: Of 576 patients in the SENSCIS trial, 150 participated in the HRCT sub-study, of whom 117 had data available on esophageal distension at baseline. Of these, 85 (72.6%) had both esophageal distension and GERD, 25 (21.4%) had esophageal distension and no GERD, and 7 (6.0%) had GERD and no esophageal distension. Compared with patients with esophageal distension but no GERD, the subgroup of patients with both esophageal distension and GERD had a greater proportion of patients with diffuse cutaneous SSc, worse modified Rodnan skin scores, greater extent of fibrotic ILD on HRCT, worse lung function, and worse quality of life based on the St George’s Respiratory Questionnaire (SGRQ) total score (Table 1). Of 116 patients with data available on esophageal diameter at baseline, 75 (64.7%) had a widest esophageal diameter >15mm. Compared with patients with a smaller esophageal diameter, the subgroup of patients with a widest esophageal diameter >15mm had a greater proportion of patients with GERD, greater proportion of patients with diffuse cutaneous SSc, worse modified Rodnan skin scores, greater extent of fibrotic ILD on HRCT, worse lung function, and worse quality of life based on the SGRQ total score (Table 2).
Conclusion: Exploratory analyses of data from a sub-study of the SENSCIS trial suggest that the combination of esophageal involvement and GERD, and a widest esophageal diameter >15 mm, were associated with worse disease severity in patients with SSc-ILD.
To cite this abstract in AMA style:
Kreuter M, Khanna D, Ryerson C, Humphries S, Takeuchi T, Hamblin M, Wormanns D, Ittrich C, Risse F, Alves M, Lynch D. Esophageal Involvement and Gastroesophageal Reflux Disease in Patients with SSc-ILD: Data from a Sub-Study of the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/esophageal-involvement-and-gastroesophageal-reflux-disease-in-patients-with-ssc-ild-data-from-a-sub-study-of-the-senscis-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/esophageal-involvement-and-gastroesophageal-reflux-disease-in-patients-with-ssc-ild-data-from-a-sub-study-of-the-senscis-trial/