Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic sclerosis (SSc) is a rare autoimmune disorder with chronic morbidity and high mortality. Disease pathogenesis involves microvascular damage, immune dysregulation, and fibrosis affecting skin and internal organs. There are no FDA approved antifibrotic treatments for skin disease. Tyrosine kinase inhibitors have demonstrated antifibrotic effects on SSc skin in clinical trials with variable efficacy and poor patient tolerance. Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase whose circulating ligands correlate with modified Rodnan skin score (mRSS) as part of a recently identified gene expression signature specific for SSc skin. EGFR activates multiple downstream signaling pathways, including PI3K/AKT (phosphatidylinositol 3-kinase, protein kinase B), MAPK (mitogen-activated protein kinase), and JAK/STAT (janus kinase/signal transducer and activator of transcription) pathways. The purpose of this project was to identify activation of EGFR and which of its downstream pathways occur in SSc patient skin.
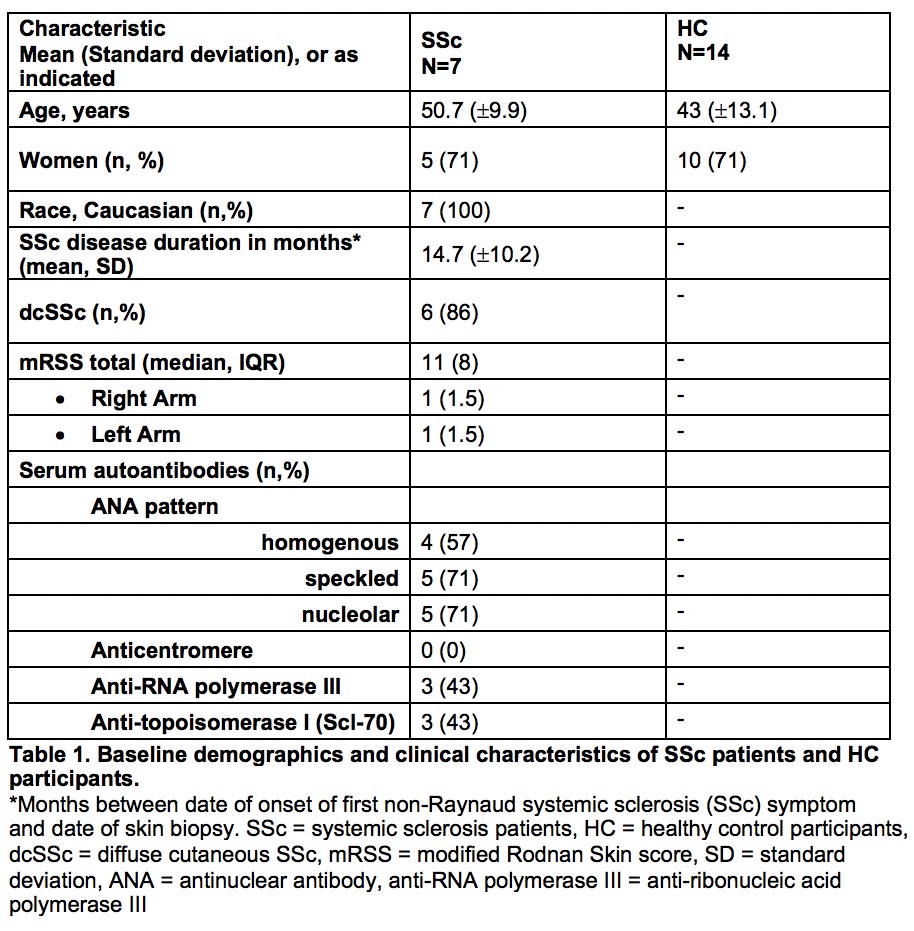
Methods: Archived forearm skin biopsies from seven SSc patients who met the 2013 ACR/EULAR classification criteria and 14 matched healthy control participants (HC) were subjected to immunohistochemical (IHC) staining using antibodies to EGFR, phospho-EGFR (p-EGFR), and downstream transcription factors AKT, phospho-AKT (pAKT), ribosomal protein S6 (S6), phospho-S6 (pS6), signal transducer and activator of transcription 1 (STAT1), and phospho-STAT1 (pSTAT1). To focus on fibroblasts, non-vascular positively stained cells were enumerated by counting the number per high powered field (hpf) in the dermis. Outcomes measures also included correlation with mRSS for each SSc patient. Statistical analysis was done using based comparison around the two groups (SSc and HC), clustered at the patient level. Clustered spearman correlation was performed for IHC and mRSS.
Results: Baseline demographics and clinical characteristics of SSc patients and HCs are shown in Table 1. Among the skin biopsies, there was 1.3-fold higher staining for pEGFR (p=0.008), 1.41-fold higher S6 (p< 0.00), and 1.44-fold higher STAT1 (p< 0.00) in SSc dermis compared to HC biopsies. There were no statistically significant differences in staining for EGFR, AKT, pAKT, and pS6 (Table 2). We did not detect phosphorylation of STAT1 by IHC in the SSc samples. pEGFR staining showed an inverse correlation with mRSS.
Conclusion: The EGFR pathway appears activated in SSc patients compared to HCs as evidenced by increased staining of its phosphorylated protein. EGFR activation may reflect extent or stage of disease based on its inverse correlation with mRSS. More fibroblasts expressed STAT1 and S6 protein in SSc skin, which suggests a higher number of a certain fibroblast subtype. However, we did not observe differences in phosphorylation of STAT1 or S6, so if EGFR signals through these pathways, it may occur with phosphorylation of a different amino acid or through unphosphorylated protein complexes.
 Protein expression in HC participants and SSc patients (a-g) with coefficients comparing expression in HC to SSc (h). EGFR = epidermal growth factor receptor, pEGFR = phospho-epidermal growth factor receptor, AKT = protein kinase B, pAKT = phospho-protein kinase B, S6 = ribosomal protein S6, pS6 = phospho-ribosomal protein S6, STAT1 = signal transducer and activator of transcription 1, HC = healthy control participants, SSc = systemic sclerosis patients
Protein expression in HC participants and SSc patients (a-g) with coefficients comparing expression in HC to SSc (h). EGFR = epidermal growth factor receptor, pEGFR = phospho-epidermal growth factor receptor, AKT = protein kinase B, pAKT = phospho-protein kinase B, S6 = ribosomal protein S6, pS6 = phospho-ribosomal protein S6, STAT1 = signal transducer and activator of transcription 1, HC = healthy control participants, SSc = systemic sclerosis patients
 a) pEGFR HC epidermis and dermis, 0 cells per high-power field b) pEGFR HC dermis, 1 cells per high-power field c) pEGFR SSc epidermis and dermis, 0 cells per high-power field d) pEGFR SSc dermis, 3 cell per high-power field. HC = healthy control participant, SSc = systemic sclerosis patient, pEGFR = phospho-epidermal growth factor receptor
a) pEGFR HC epidermis and dermis, 0 cells per high-power field b) pEGFR HC dermis, 1 cells per high-power field c) pEGFR SSc epidermis and dermis, 0 cells per high-power field d) pEGFR SSc dermis, 3 cell per high-power field. HC = healthy control participant, SSc = systemic sclerosis patient, pEGFR = phospho-epidermal growth factor receptor
To cite this abstract in AMA style:
Carnaru M, Hinchcliff M, Odell I, Wilson F. Epidermal Growth Factor Receptor Pathway and Fibrosis in Systemic Sclerosis Skin [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/epidermal-growth-factor-receptor-pathway-and-fibrosis-in-systemic-sclerosis-skin/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/epidermal-growth-factor-receptor-pathway-and-fibrosis-in-systemic-sclerosis-skin/