Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: EP-104IAR is being developed to treat OA symptoms. Previous results from non-clinical studies evaluating local joint safety in beagle dogs, in contrast to other corticosteroid preparations, indicated that the prolonged local residence time of EP-104IAR had no impact on cartilage health.1 Safety and PK data from a Phase 1 trial in 24 OA knee patients were consistent with nonclinical findings and supported continued development of EP-104IAR.2 This report describes the results of a Phase 2b trial in subjects with Knee OA.
Methods: Subjects were randomized 1:1 to a single intraarticular dose of EP-104IAR 25 mg, or vehicle and followed for 24 weeks. Males and females, ≥40 years, with a diagnosis of primary knee OA, Kellgren-Lawrence Grade 2-3 and symptoms (WOMAC Pain scores ≥ 4.0 to ≤9.0 (out of 10)) were enrolled. Subjects recorded weekly WOMAC Pain and monthly WOMAC Total measurements on a diary device. Safety assessments included adverse events (AEs), vital signs, laboratory evaluations, and knee examinations. The primary endpoint was the change from baseline between treatments in WOMAC® Pain at Week 12.
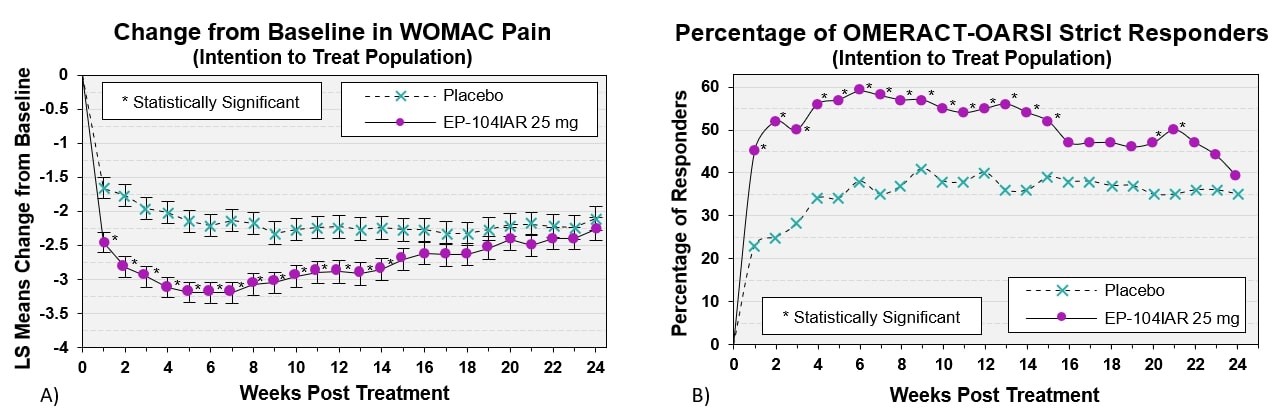
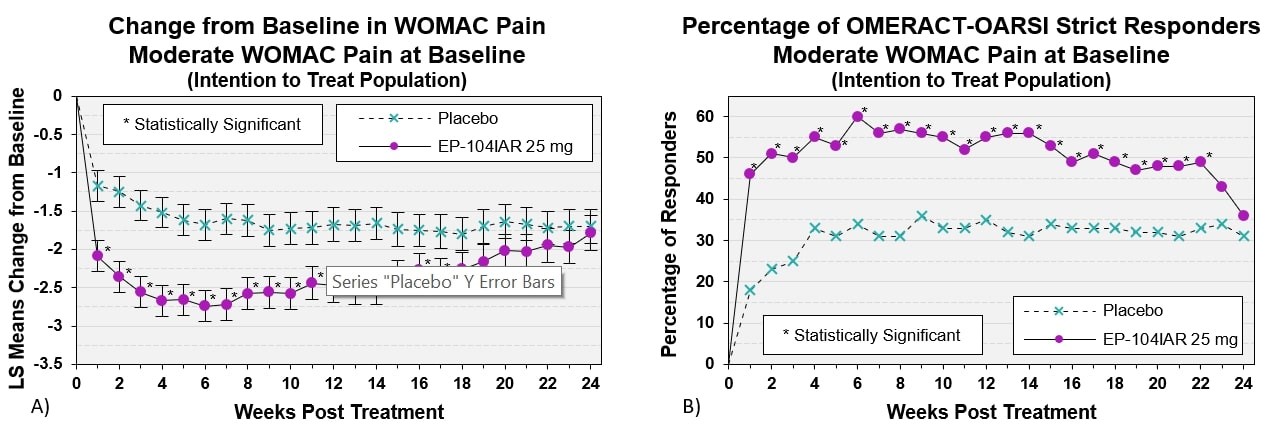
Results: 318 subjects were dosed (163 EP-104IAR, 155 vehicle), median age = 64 years, 57.5% female. 68% had moderate OA pain (average baseline WOMAC Pain 3.5-6.5). Participants treated with EP-104IAR had significantly higher pain relief compared to vehicle in WOMAC Pain at Week 12 (change from baseline: -2.89 EP-104IAR, -2.23 vehicle; 95%CI -1.1, -0.2, p=0.004). This significant difference persisted until Week 14 in the full population and until Week 17 in subjects with moderate pain. A similar pattern was observed in the OMERACT-OARSI Strict Responders analysis: in allcomers % of OMERACT-OARSI Strict Responders was statistically different until Week 15 while in moderates there was statistical significance to Week 22 (both p< 0.05).Most AEs were mild/moderate. There were no EP-104IAR-related serious AEs or withdrawals. Mean serum cortisol and glucose values were similar in both treatment arms, no subjects developed adrenal insufficiency.
Conclusion: A single dose of EP-104IAR provided clinically and statistically significant pain relief for 14 weeks compared to vehicle. In subjects with moderate pain, significant pain-relief persisted for 17 weeks. Responder analyses in subjects with moderate pain demonstrated that clinically meaningful differences persisted for the majority of the study. This suggests EP‑104IAR could offer clinically meaningful and safe benefit for substantially longer than any other currently marketed corticosteroids.
To cite this abstract in AMA style:
Helliwell J, Malone A, Kowalski M, Bihlet A, Miller C, Mondragon A, Li Y, Dobek C, Peck V, Wilmink M, Simon L. EP-104IAR (Extended-Release Fluticasone Propionate for Injectable Suspension): Topline and Key Secondary Results from a Phase 2 Randomized, Double-blind, Vehicle-Controlled Trial in Subjects with Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/ep-104iar-extended-release-fluticasone-propionate-for-injectable-suspension-topline-and-key-secondary-results-from-a-phase-2-randomized-double-blind-vehicle-controlled-trial-in-subjects-with-knee/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ep-104iar-extended-release-fluticasone-propionate-for-injectable-suspension-topline-and-key-secondary-results-from-a-phase-2-randomized-double-blind-vehicle-controlled-trial-in-subjects-with-knee/