Session Information
Date: Tuesday, November 14, 2023
Title: (2019–2038) Patient Outcomes, Preferences, & Attitudes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Healthcare research has historically been medically oriented, less focused on the patients’ perspective that research shows can improve the quality of care. OMERACT advocates for the development of core outcome sets to improve outcome measures in rheumatology by incorporating the patient perspective, which is often neglected in research. The OMERACT Glucocorticoid (GC) Impact Working Group has been working to develop a core domain set to measure the impact of GCs. Based on qualitative research and multiple rounds of Delphi-type exercises, the group identified mandatory domains for inclusion in all clinical trials where the effects of GCs are measured. These include infections, bone fragility, mood disorders, hypertension, diabetes, weight, fatigue, and mortality.
A fundamental aim of the GC Impact Working Group is to develop a core outcome set of measurement tools or instruments to represent and assess relevant outcomes, through application of the well-established OMERACT methodology. Before progressing to instrument selection, the Working Group sought to establish precise definitions of all mandatory domains within the core domain set to facilitate measurement.
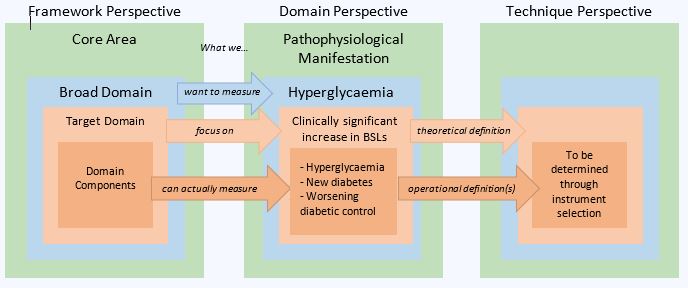
Methods: OMERACT methodology was applied with the use of evidence and consensus-based decision making of all stakeholder groups (patient research partners, health care professionals, clinician researchers, industry members and methodologists) to develop detailed definitions for the broad domain, target domain, and domain components, considering sources of variability that could impact the outcome measure assessed by a given instrument (fig 1). The working group synthesized prior qualitative studies, quantitative work, and results from Delphi rounds, to develop a rich definition of ‘what’ is to be measured.
Results: Over a 2-year period, from 2021–2023, the OMERACT Working Group on GC Impact conducted virtual meetings to establish domain definitions. The core area identified for all domains was pathophysiological, except weight, which was divided into both pathophysiological and life impact manifestations, and fatigue which has only life impact manifestations. Sources of variability were recognized, including cultural factors, age, gender, education level, socioeconomic status, personal experiences, emotional state, and language barriers. To minimize the impact of potential variability, instrument selection will require rigorous validation procedures. The domain definitions endorsed, through this consensus-based decision-making process, will form the foundation for instrument selection and the initial step of domain / concept match and content validity in the OMERACT pillar of ‘truth’ before moving on to feasibility, and discrimination
Conclusion: The OMERACT GC Impact Working Group have endorsed detailed domain definitions for core domains. The next step of the working group is to select instruments and develop the core outcome set for inclusion in all clinical trials where the effects of GCs are measured.
To cite this abstract in AMA style:
Yip K, Lyne S, Vasiliou V, Katz D, Richards P, Tieu J, Black R, Bridgewater S, Beaton D, maxwell l, Robson J, Mackie S, Hill C, Goodman S. Endorsement of Core Domain Definitions to Measure the Impact of Glucocorticoids in Patients with Rheumatic Diseases: A Report from the OMERACT Working Group on Glucocorticoid Impact [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/endorsement-of-core-domain-definitions-to-measure-the-impact-of-glucocorticoids-in-patients-with-rheumatic-diseases-a-report-from-the-omeract-working-group-on-glucocorticoid-impact/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/endorsement-of-core-domain-definitions-to-measure-the-impact-of-glucocorticoids-in-patients-with-rheumatic-diseases-a-report-from-the-omeract-working-group-on-glucocorticoid-impact/