Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
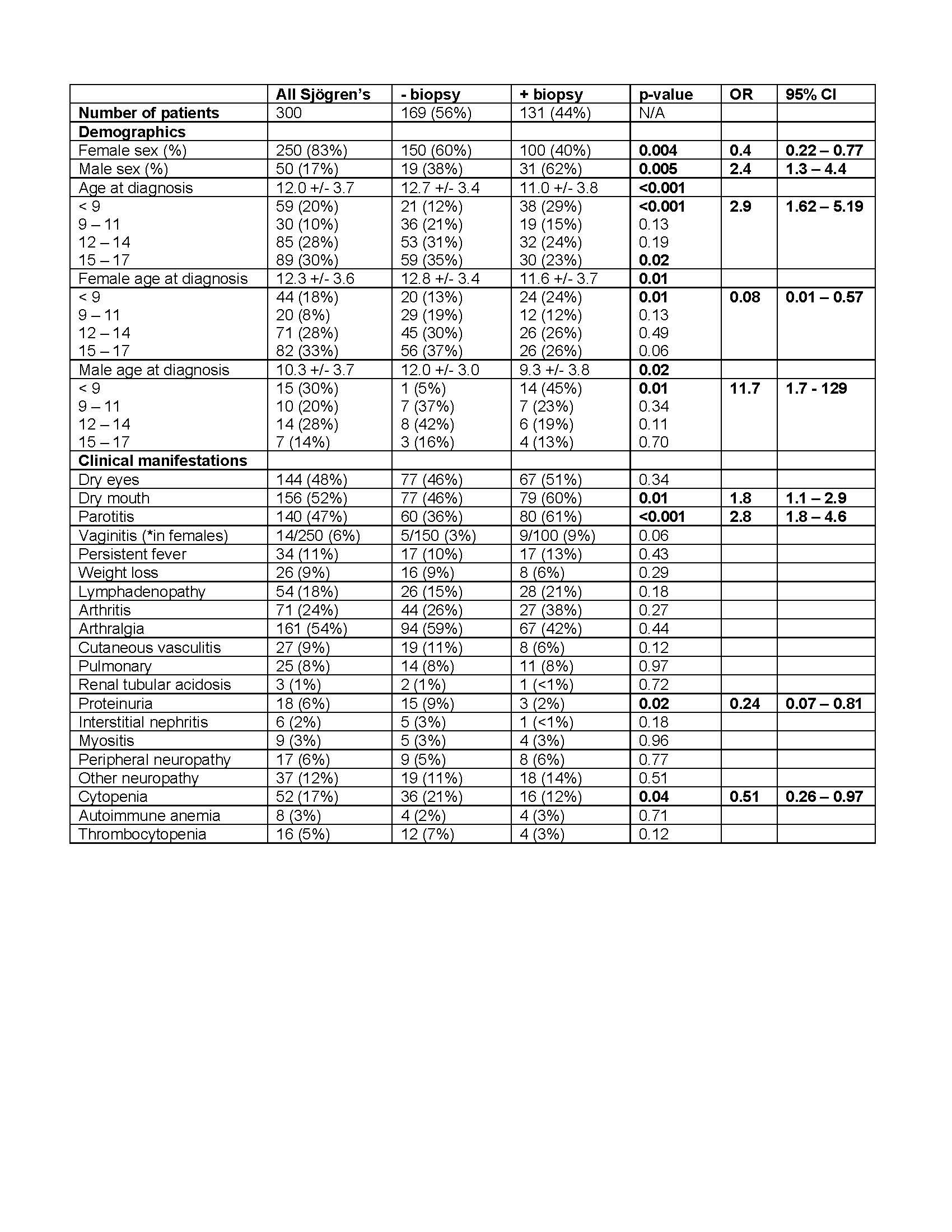
Background/Purpose: Sjögren’s disease is a rare condition in children. Diagnosis is often delayed because of the lack of pediatric-specific guidelines for childhood Sjögren’s disease (cSjD). In a retrospective cohort generated by the Childhood Sjögren’s Disease Workgroup, minor salivary gland (MSG) biopsy was performed as part of the evaluation for cSjD in 56% of the patients (Basiaga et al), suggesting the presence of factors which influence how MSG biopsy is utilized in the evaluation of cSjD.
Methods: Using the retrospective international cohort of 300 children diagnosed with cSjD, we compared demographics, clinical manifestations, disease features, and diagnostic evaluation of cSjD patients who underwent MSG biopsy as part of their evaluation of cSjD to those who did not.
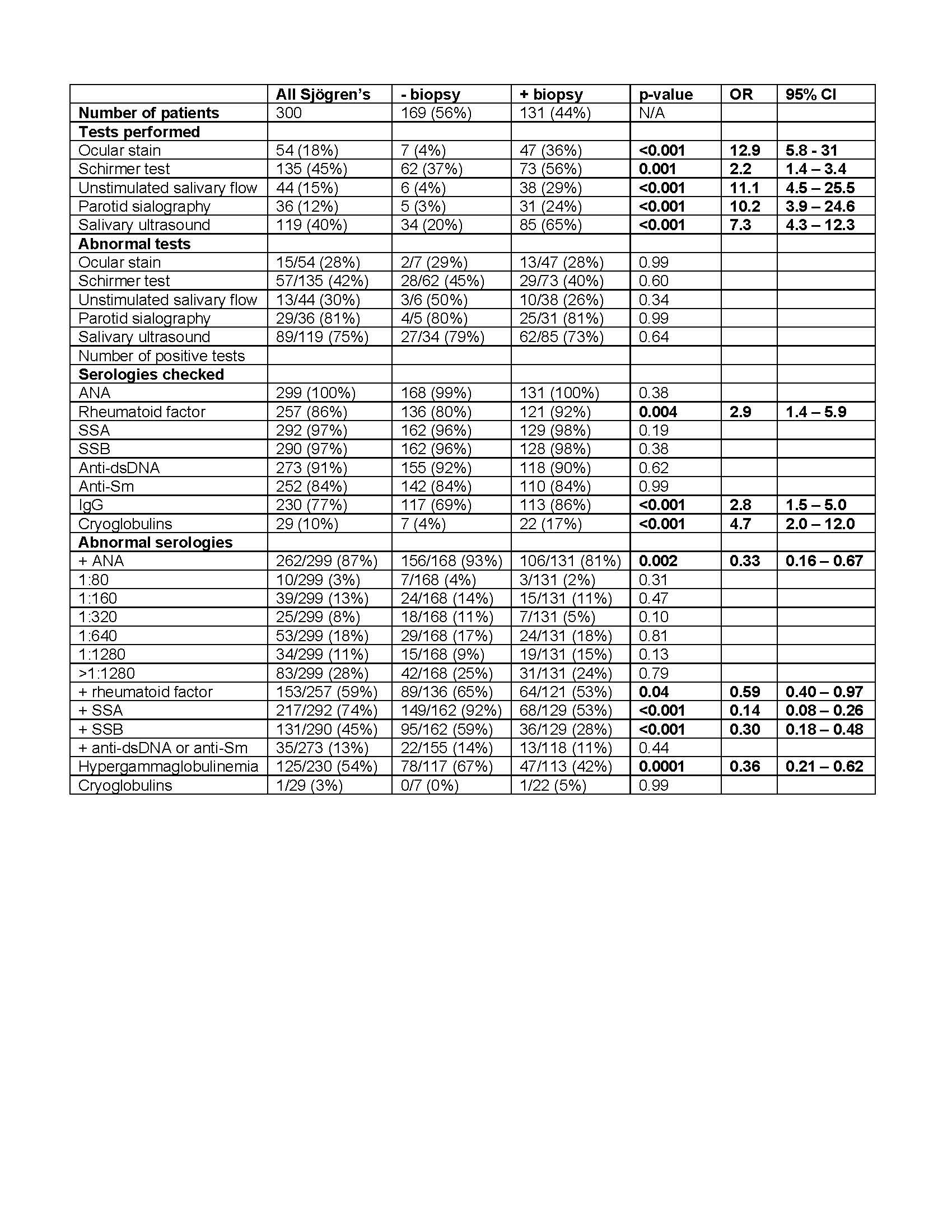
Results: We found that younger age (less than 9 years old, p=0.0003, OR 2.9), male biologic sex (p=0.005, OR 2.4), parotitis (p< 0.0001, OR 2.8), and dry mouth (p=0.01, OR 1.8) were associated with a higher likelihood of MSG biopsy (Table 1). Hypergammaglobulinemia (p=0.0001, OR 0.36) and serologies positive for SSA (p < 0.0001, OR 0.14), SSB (p< 0.0001, OR 0.30), RF (p=0.04, OR 0.59), and ANA (p=0.002, OR 0.33), all of which strongly support cSjD, were associated with a lower likelihood of MSG biopsy (Table 2). The presence of cytopenia and proteinuria were significantly increased in patients diagnosed with cSjD who did not undergo MSG biopsy (Table 2). To examine how variation in provider practice influenced the use of MSG biopsy, we stratified patients according to contributing provider, which interestingly revealed a bimodal distribution of usage of MSG biopsy, supporting a wide variation in the use of MSG biopsy for the evaluation of cSjD. In contrast to providers who routinely evaluated with MSG biopsy, those who did not routinely evaluate with MSG biopsy also had reduced use of other diagnostic evaluations, including Schirmer test (p=0.001), ocular staining score (p< 0.0001), unstimulated salivary flow (p< 0.0001), and salivary gland ultrasound (p< 0.0001), further supporting the wide practice variation in the evaluation of cSjD.
Conclusion: Taken together, these findings suggest that a standardized evaluation of cSjD is needed to improve diagnosis and to better understand the epidemiology and natural history of this rare clinical entity, and ultimately provide the foundation for clinical trials for the treatment of cSjD.
References:
Basiaga, ML et al. Childhood Sjögren’s Syndrome: features of an international cohort and application of the 2016 ACR/EULAR classification criteria. Rheumatology, Volume 60, Issue 7, July 2021, Pages 3144–3155.
To cite this abstract in AMA style:
Brian D, Basiaga M, Stern S, Thatayatikom A, cha s, Lieberman S, Srinivasalu H, CARRA and the childhood Sjogren's workgroup o. Elucidating the Factors That Influence the Use of Minor Salivary Gland Biopsy for the Evaluation of Childhood Sjögren’s Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/elucidating-the-factors-that-influence-the-use-of-minor-salivary-gland-biopsy-for-the-evaluation-of-childhood-sjogrens-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/elucidating-the-factors-that-influence-the-use-of-minor-salivary-gland-biopsy-for-the-evaluation-of-childhood-sjogrens-disease/