Session Information
Date: Sunday, October 26, 2025
Session Type: Abstract Session
Session Time: 3:15PM-3:30PM
Background/Purpose: Anti-Cyclic Citrullinated Peptide (anti-CCP) antibodies are one of the strongest risk factors for the development of rheumatoid arthritis (RA). Anti-CCP antibodies are generated against citrullinated proteins, which arise when peptidylarginine deiminases (PADs) make post-translational modifications to proteins by the deamination of arginine residues to citrulline. PAD2 and PAD4 isomer expression is associated with RA and serum anti-PAD4 antibodies are strongly associated with anti-CCP antibody positivity and aggressive disease phenotype. However, the specific role of serum PAD4 in anti-CCP antibody positive (CCP+) at-risk individuals who do not have synovitis, and its relationship with arthritis development, is unclear.Our objectives were to (i) investigate baseline serum PAD2/4 levels in CCP+ at-risk individuals with musculoskeletal symptoms but no synovitis, compared with CCP+ early RA patients and healthy controls and (ii) to investigate the relationship between serum PAD2/4 levels and future arthritis development.
Methods: Stored serum samples were selected from the Leeds CCP study. We selected: (i) CCP+ at-risk individuals with high anti-CCP antibody levels ( >100 U/ml) and Early Morning Stiffness (EMS) lasting ≥30 minutes (n=79) (ii) early, treatment-naïve RA patients (n=34) and (iii) asymptomatic healthy controls (n=44). Follow-up data was collated for all CCP+ at-risk individuals to determine future arthritis progression status. PAD2/4 levels were assayed via Enzyme-linked Immunosorbent Assay (ELISA), performed in triplicate, following manufacturer’s instructions, Cayman Chemicals PAD2 (Item No. 501450) and PAD4 (Item No. 501460). All results were analysed using R (version 4.3.1).
Results: All groups were balanced for age, sex and smoking status (Table 1). Anti-CCP antibody levels and duration of EMS were similar for CCP+ at-risk individuals and early RA patients (Table 1). PAD4 but not PAD2 levels were higher in early RA patients compared with healthy controls (p=0.00922). There was no statistically significant difference in PAD4 levels between CCP+ at-risk individuals and healthy controls (p=0.084). However, when CCP+ at-risk individuals were separated according to future arthritis progression status, a significant increase in PAD4 levels in future progressors (n=50) compared to both future non-progressors (n=29) (p=0.022) and healthy controls (p=0.01) was observed (Figure 1). PAD4 levels were similar in healthy controls and future non-progressors (p=0.888) as well as in future progressors and early RA patients (p=0.975). There was no statistically significant difference in PAD2 levels between any of the groups.
Conclusion: These data suggest serum PAD4 levels are increased in CCP+ at-risk individuals who progress to RA compared with CCP+ future non-progressors and healthy controls. Serum PAD4 levels are also similar in Pre-RA and RA. PAD4 levels may therefore have utility in predicting arthritis development and PAD4 may also be a potential therapeutic target for arthritis prevention in those at high risk.
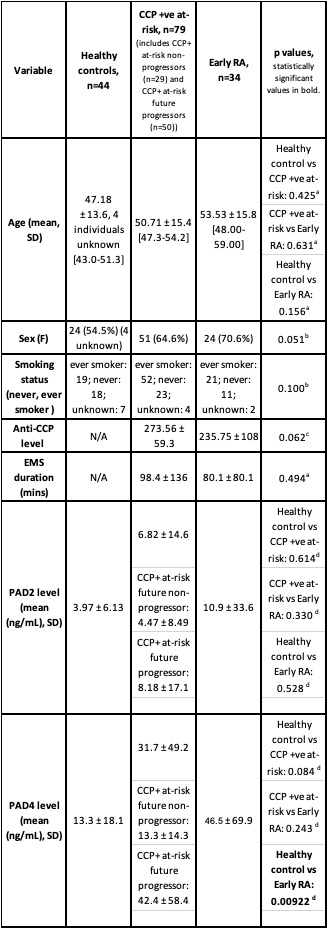 Table 1 – Comparison of demographic, clinical and biomarker data between the three patient groups.
Table 1 – Comparison of demographic, clinical and biomarker data between the three patient groups.
aANOVA test b Fisher’s Exact Test c t-test d Wilcoxon rank sum test. Definitions: EMS: Early morning stiffness; CCP
titre: 2nd generation anti-Cyclic Citrullinated Peptide antibody titre; PAD2: peptidylarginine deiminase 2; PAD4:
peptidylarginine deiminase 4; F: Female; M: Male.
.jpg) Figure 1 – PAD2 and PAD4 levels according to patient group. Plot completed via Wilcoxon rank sum test with
Figure 1 – PAD2 and PAD4 levels according to patient group. Plot completed via Wilcoxon rank sum test with
no additional covariate adjustments. Definitions: ns: no statistical significance; * statistical significance at
p < 0.05; **: statistical significance at p < 0.01; ***: statistical significance at p < 0.001.
To cite this abstract in AMA style:
Sharrack S, Yu X, Jayaraman A, Duquenne L, Di Matteo A, Harnden K, Thornton L, Jayne-Sugden H, Nam J, Smith A, Collins M, Sparks R, Jiang F, Konstantinidis K, Neisen J, De Baets G, Close D, chamberlain C, Platt A, Emery P, Meade J, Mankia K. Elevated serum peptidylarginine deiminase 4 (PAD4) levels in anti-CCP antibody positive at-risk individuals with arthralgia who progress to rheumatoid arthritis. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/elevated-serum-peptidylarginine-deiminase-4-pad4-levels-in-anti-ccp-antibody-positive-at-risk-individuals-with-arthralgia-who-progress-to-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/elevated-serum-peptidylarginine-deiminase-4-pad4-levels-in-anti-ccp-antibody-positive-at-risk-individuals-with-arthralgia-who-progress-to-rheumatoid-arthritis/
