Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Chronic pain is an unmet need in osteoarthritis (OA) as current therapies have limited analgesia and side effects. The chemokine CCL17 mediates inflammatory pain and blocking CCL17 (via anti-CCL17 monoclonal antibodies [mAb] or CCL17 knock-out) reduces pain and joint disease in murine arthritis. GSK3858279 is a novel, high affinity, human mAb that functionally inhibits CCL17. The objective of this study is to present efficacy, safety, pharmacokinetic (PK) and immunogenicity data from Part B of a first-in-human, 2-part, phase I, randomized, double-blind, placebo (PBO)-controlled study evaluating GSK3858279 for OA pain (NCT03485365).
Methods: Participants with knee OA per ACR criteria, Kellgren and Lawrence (KL) score ≥2 and average daily pain score 4–9 by 11-point numerical rating scale (NRS), were randomized (1:1) to weekly subcutaneous (SC) GSK3858279 or PBO for 8 weeks. Co-primary endpoints were change from baseline (CFB) to Week 8 in average and worst knee pain intensity. Participants completed a daily pain NRS. Pain medication was prohibited, except paracetamol (≤3g/day) as rescue medication (not 24h before a study visit). Exploratory endpoints included CFB in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores. Samples for analysis of GSK3858279 serum concentration and immunogenicity were collected to Week 20. A Bayesian repeated measures model using non-informative priors was fitted to CFB data; 95% credible intervals (CrI) for treatment differences were calculated.
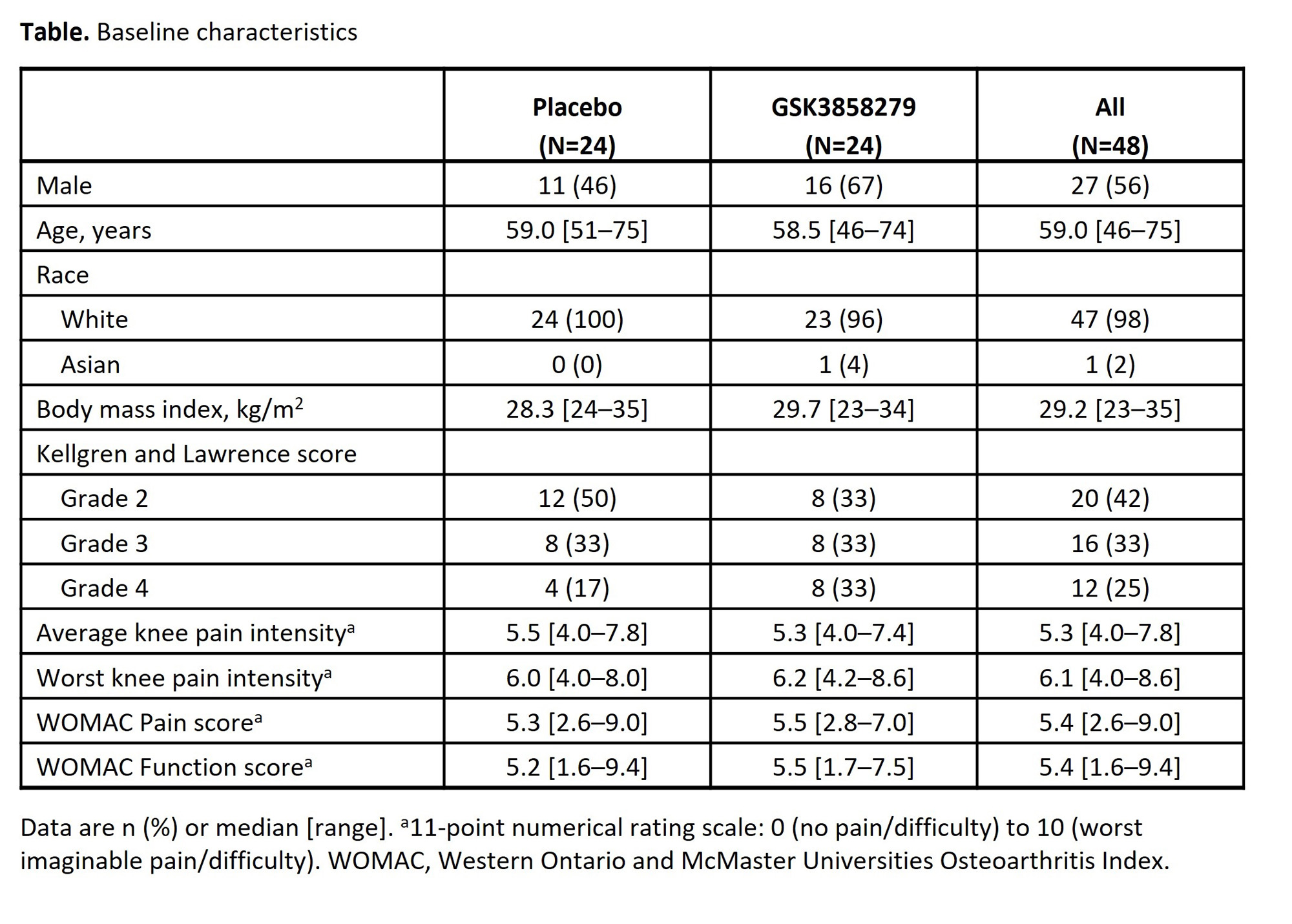
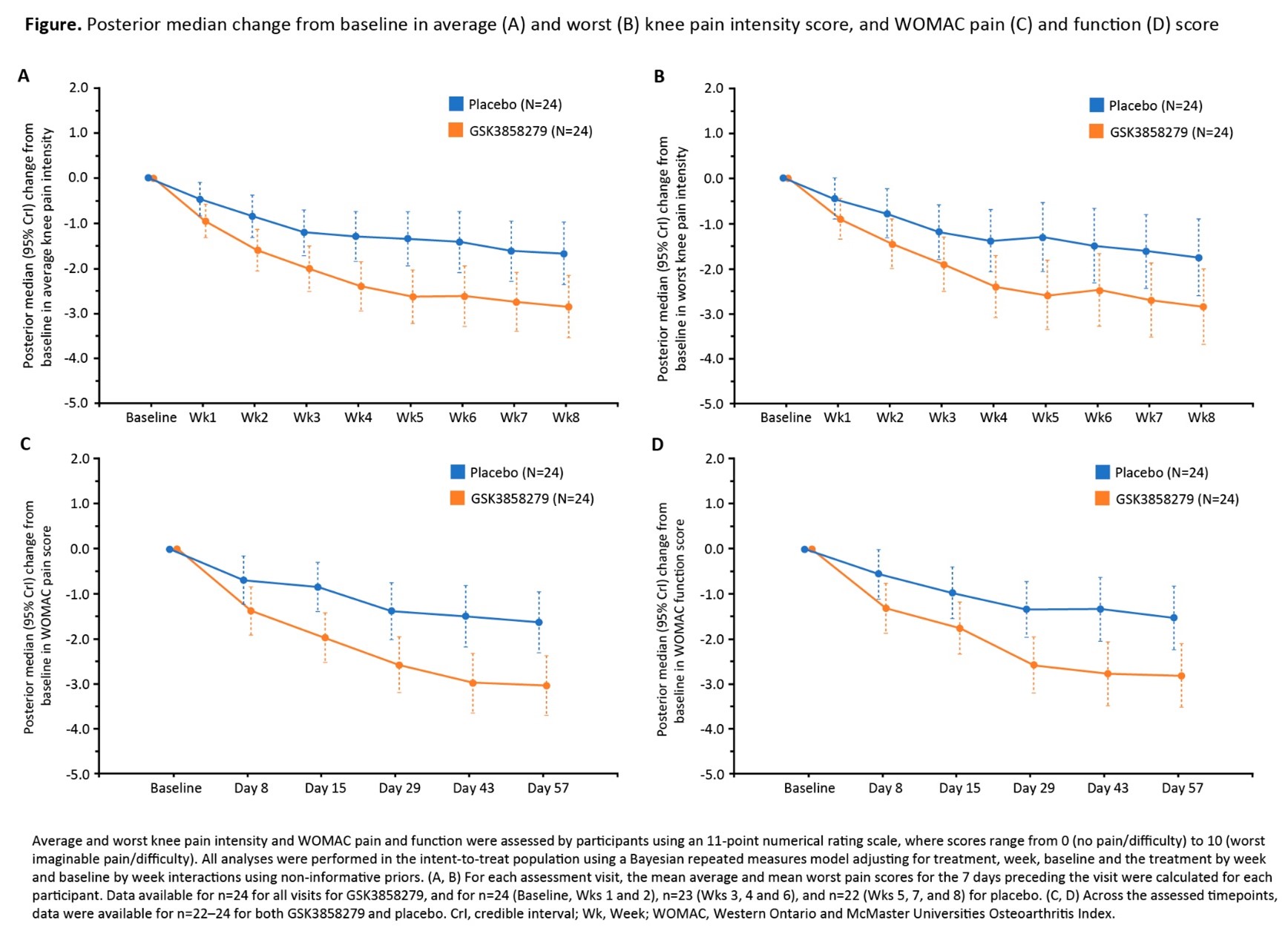
Results: All 48 participants in Part B completed the study (2 participants in the PBO arm discontinued treatment). Participant characteristics were comparable; however, more participants in the GSK3858279 arm were male and KL grade 4 (Table). GSK3858279 was rapidly absorbed after SC administration (median Tmax ~2 days), with steady concentrations predicted by Week 8. Anti-GSK3858279 Abs were detected in 5/24 (21%) and 1/24 (4%) participants who received GSK3858279 and PBO, respectively; PK profiles for these participants were consistent with profiles in those without anti-drug Abs. For the co-primary endpoints of average and worst knee pain intensity, GSK3858279 showed greater improvement versus PBO at all timepoints (Figure). At Week 8, the difference (95% CrI) in CFB for GSK3858279 versus PBO was −1.18 (−2.15, −0.20) and −1.09 (−2.29, 0.12) for average and worst knee pain intensity, respectively (probability of true difference < 0: >99% and 96%). GSK3858279 showed greater improvement in WOMAC pain and function scores versus PBO (Figure). At Day 57, the difference (95% CrI) in CFB for GSK3858279 versus PBO was −1.41 (−2.35, −0.46) for pain and −1.29 (−2.28, −0.29) for function (probability of true difference < 0: >99% for both). Adverse events (AE) occurred in 21/24 (88%) and 15/24 (63%) participants, GSK3858279 and PBO, respectively. There were no serious AEs or deaths.
Conclusion: Weekly dosing for 8 weeks with GSK3858279 has clinical activity (improved pain scores) and a favourable safety profile in patients with OA knee pain. These compelling data warrant further study of the effectiveness and safety of GSK3858279 in people with OA pain.
To cite this abstract in AMA style:
Singh Nijjar J, Abbott-Banner K, Ray R, Munoz Vicente S, Bentley J, Muya C, Siederer S, Panoilia E, Bass D, Fernando D, Emery E. Efficacy, Safety, Pharmacokinetics and Immunogenicity of Repeated Dosing of GSK3858279 in Patients with Knee Osteoarthritis: A Phase I, Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-safety-pharmacokinetics-and-immunogenicity-of-repeated-dosing-of-gsk3858279-in-patients-with-knee-osteoarthritis-a-phase-i-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-safety-pharmacokinetics-and-immunogenicity-of-repeated-dosing-of-gsk3858279-in-patients-with-knee-osteoarthritis-a-phase-i-randomized-double-blind-placebo-controlled-study/