Session Information
Date: Monday, October 27, 2025
Title: (1123–1146) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Pozdeutinurad (AR882, POZD) is a novel, selective URAT1 inhibitor currently in phase 3 clinical stage development for the treatment of gout and tophaceous gout. To date, only intravenous (IV) therapies such as pegloticase is indicated in urate-lowering (ULT) refractory patients and has demonstrated tophi dissolution by caliper measurement and Dual Energy Computer Tomography (DECT). However, IV therapy is not available or appropriate for the majority of patients given infusion costs, drug availability and potential toxicities from necessary use with concomitant immunosuppressives. POZD is an oral agent being evaluated for both urate control and dissolution of subcutaneous tophi. This analysis explores the distinction between POZD 75 mg monotherapy or in combination with allopurinol in tophaceous gout patients who are either treatment naïve or suboptimally controlled.
Methods: The phase 2 trial recruited 42 patients with at least one subcutaneous tophus. Patients were eligible if they were either inadequately controlled on ULT with serum urate (sUA) > 6.0 mg/dL or were ULT naïve with sUA ≥ 7.0 mg/dL. After ULT washout (if applicable), patients were randomized to receive POZD 75 mg, POZD 50 mg + allopurinol, or allopurinol up to 300 mg. At 6 months all patients were able to opt into two subsequent 6-month extensions with treatment modifications. sUA, tophi measurements with calipers or urate crystal measurement using DECT were collected up to 18 months. Results for sUA response rates, complete resolution in target tophus and reduction in urate crystal volumes were analyzed.
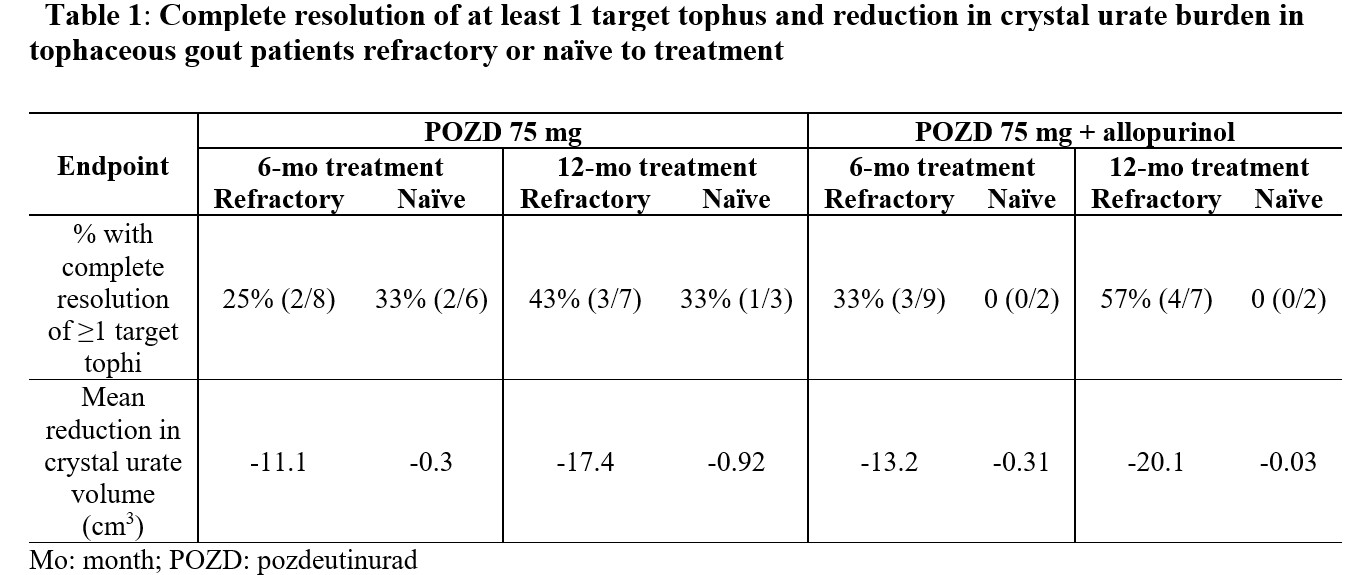
Results: Patients refractory to their pre-study ULT had a higher baseline sUA (9.5 to 9.8 mg/dL) than treatment naïve patients (7.6 to 8.5 mg/dL). Both populations had clinically significant and sustained sUA responses following treatment. In the 75 mg group, refractory patients had a 49% mean decrease in sUA levels, while the naïve patients achieved a 54% decrease. In the 75 mg + allopurinol group, the refractory patients had a 61% mean decrease in sUA levels, while the naïve patients achieved a 74% decrease. At 12 months, refractory patients demonstrated complete resolution of at least 1 target tophus in 43% and 57% of patients in the 75 mg group, and in 75 mg + allopurinol group, respectively. Although all ULT naïve patients had at least a partial response, only the 75 mg group had at least 1 target tophus fully resolved at 33% (Table 1). The refractory patients demonstrated greater reduction in their urate crystal burden compared to ULT naïve patients following 12 months treatments (-17.4 vs -0.92 cm3 for 75 mg; -20.1 vs -0.03 cm3 for 75 mg + allopurinol treatment) (Table 1).
Conclusion: Pozdeutinurad 75 mg monotherapy or in combination with allopurinol in tophaceous patients refractory and naïve to ULT were effective in sUA lowering, demonstrating high rates of complete resolution of target tophus and reduction in urate crystal burden. Pozdeutinurad alone or combination may provide an effective treatment option for patients with chronic tophi and inadequate response to their current treatment regimens.
To cite this abstract in AMA style:
Keenan R, Khanna P, Shen Z, Morris S, Mundell P, Wei W, Polvent E, Hingorani V, Yan S, Yeh L. Efficacy of Pozdeutinurad (AR882) in Treatment Naïve and Suboptimally Treated Gouty Arthritis with Tophi [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-pozdeutinurad-ar882-in-treatment-naive-and-suboptimally-treated-gouty-arthritis-with-tophi/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-pozdeutinurad-ar882-in-treatment-naive-and-suboptimally-treated-gouty-arthritis-with-tophi/