Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) comprise a heterogenous group of acquired autoimmune diseases characterised by inflammation of muscle and affection of other organs, including lung and skin. Some cases of IIM are non-responsive to conventional treatment with glucocorticoids and DMARDs requiring treatment escalation. Only limited data on efficacy and safety of immune-apheresis (IA) in IIM patients exist. The aim of this study was to investigate the efficacy and safety of IA in therapy-refractory IIM.
Methods: Patients with highly active IIM undergoing IA (either plasma-exchange or immunoadsorption) at the Medical University Vienna were included in this retrospective cohort study. Patient characteristics and clinical data including serum levels of creatine kinase and concomitant medication were extracted from electronic medical records. As a primary endpoint, efficacy of IA was evaluated four weeks after initiation of IA, calculating absolute and relative change of serum creatine-kinase (CK)-values as well as changes in steroid dose. Secondary endpoints included absolute and relative changes of CK-values at week 8 and week 12.
Results: From 2000 to 2021 25 IIM patients treated with IA were identified, 24 could be used for further analyses. Patient characteristics at start of IA are shown in Table 1. Subtypes of IIM included dermatomyositis (DM 54.2%), polymyositis (PM 8.3%), overlap-myositis (20.8%), immune-mediated-necrotising-myositis (IMNM 8.3%), and anti-synthetase syndrome (ASS 8.3%). Patients received concomitant steroid therapy (91.7%) and DMARD therapy (62.5%).
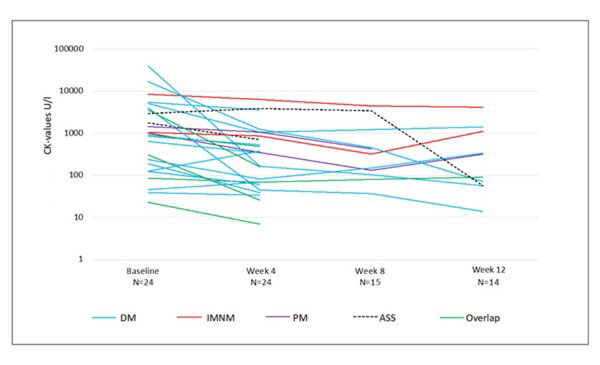
Decrease in CK-values was observed in 21/24 patients (figure 1), with significant differences between baseline (970.5 [157.5; 3795.5]U/ml) and week 4 (347 [53.5; 962] U/l). Median [IQR] dose reduction of steroids between baseline and week 4 was 12.5 [0; 12.5] mg/day absolute and 25% [0%, 100%] relative. No differences were observed within patients of different myositis subtypes. One patient died after 4 weeks; 14 patients could maintain with IA treatment until week 12. The CK-values of week 8 and 12 decreased to respectively 322 [81; 461], 101 [57; 327] U/l. Absolute and relative changes in CK-values from baseline until week 12 are displayed in table 1.
Conclusion: Immune-apheresis is associated with therapeutic benefit in refractory IIM, leading to decrease of CK-values and steroid dose.
To cite this abstract in AMA style:
Kastrati K, Rajab H, Rader A, Aichner E, Karonitsch T, Kiener H, Bonelli M, Aletaha D, Radner H. Efficacy of Immune-apheresis in Patients with Inflammatory Myopathies: A Case Series [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-immune-apheresis-in-patients-with-inflammatory-myopathies-a-case-series/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-immune-apheresis-in-patients-with-inflammatory-myopathies-a-case-series/