Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is a chronic multi-system autoimmune rheumatic disorder. Despite therapeutic advances, managing SLE remains challenging due to its complex pathogenesis and variable clinical presentations. Belimumab, a monoclonal antibody targeting aberrant B cell activity, is an approved treatment for patients with active autoantibody positive SLE.
Methods: This retrospective cohort study was conducted at a single United Kingdom centre, including patients diagnosed with SLE who received IV Belimumab. Patient demographics, disease characteristics, laboratory results and clinical outcomes were extracted from medical records. Disease activity was assessed using BILAG and SLEDAI scores, and damage accrual by SLICC at baseline, 6 months and annually. Chi-square and Fisher’s exact tests evaluated responses in specific disease manifestations after 6 months of Belimumab. Changes in immunosuppressive doses, serological markers, and ESR were evaluated using Wilcoxon signed-rank test. Mann Whitney U test evaluated significance in damage accrual between early and late initiation of Belimumab.
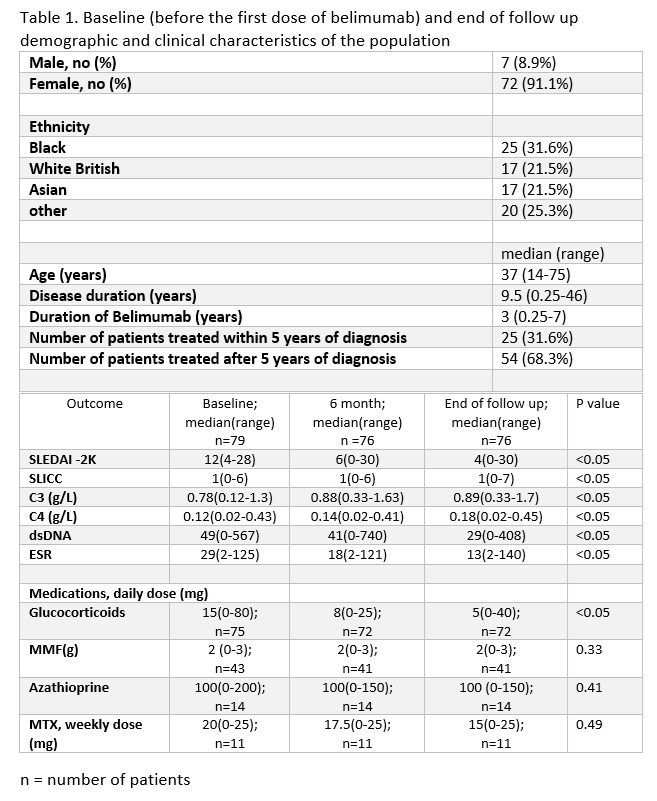
Results: 79 patients fulfilling EULAR/ACR SLE classification criteria with active SLE, characterized by elevated dsDNA and low complements (table 1) were included. 3 patients were lost to follow up.
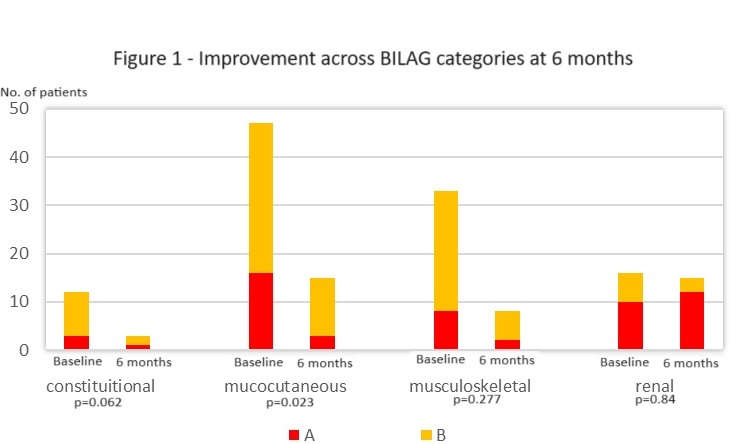
High baseline BILAG scores in mucocutaneous (20.3%-A, 40.5%-B), musculoskeletal (11.4%-A, 31.6%-B), and renal (12.7%-A, 7.6%-B) domains indicated significant systemic involvement.
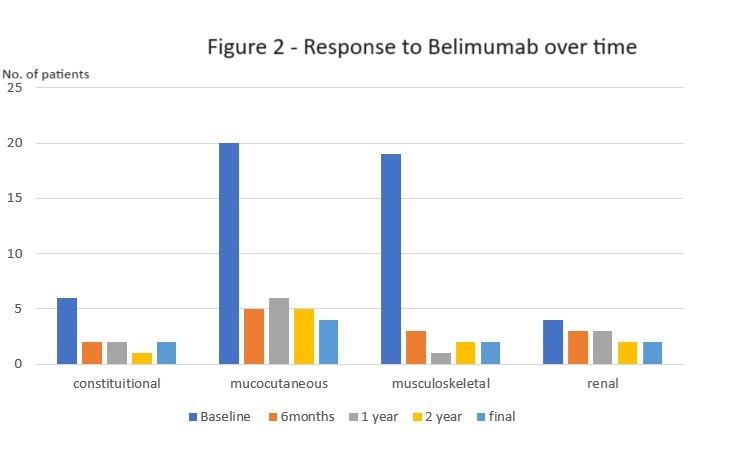
The majority of patients (64.9%) demonstrated significant SLEDAI improvement (≥4 points) within 6 months, sustained for 3+ years. BILAG improvement was defined as a change from A/B to C/D scores. High response rates were observed in constitutional (75%), musculoskeletal (75%), and mucocutaneous (68%) domains, with statistically significant improvement only in mucocutaneous symptoms (p=0.023). No improvement was observed in the renal domain.
Statistically significant improvements were observed in C3, C4, dsDNA levels and ESR. (table 1) The median daily prednisolone dose decreased significantly from 15 mg (0-80) at baseline to 5mg (0-40) at the end of follow-up (p< 0.05). Dose changes of MMF, MTX and AZA were not statistically significant.
There was no statistically significant difference in damage accrual between early (within 5 years of onset) and late initiation of belimumab. During treatment with belimumab, 21% of patients experienced a disease flare and there were no deaths.
The main reasons for the discontinuation of Belimumab were ineffectiveness (26.3%), recurrent infections (3.8%) and allergy (3.8%).
Conclusion: Belimumab improved constitutional, musculoskeletal, and mucocutaneous SLE manifestations , with statistical significance only in mucocutaneous symptoms. These improvements were supported by statistically significant improvements in serological markers (C3, C4, dsDNA, ESR) and steroid dosage. Belimumab demonstrated a significant therapeutic benefit for a substantial proportion of patients with minimal adverse effects.
To cite this abstract in AMA style:
Jayaratna b, Serasinghe P, Nel L, Jain S, Sangle S, Lopez B, Sanna G, Fernando M, DCruz D. Efficacy of Belimumab in Systemic Lupus Erythematosus: A Single-Centre Retrospective Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-belimumab-in-systemic-lupus-erythematosus-a-single-centre-retrospective-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-belimumab-in-systemic-lupus-erythematosus-a-single-centre-retrospective-study/