Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The ASAS consensus definition of ‘early axial spondyloarthritis (axSpA)’ (axial symptoms ≤ 2 years) was expert-based given the scarcity of evidence1. We conducted a meta-analysis of all placebo-randomised controlled trials (RCTs) of approved bDMARDs and tsDMARDs in patients with axSpA, with the aim of investigating the influence of symptom duration on treatment response.
Methods: AxSpA RCTs involving adult axSpA patients treated with b/tsDMARDs compared to placebo were identified from systematic literature reviews informing the ASAS-EULAR management recommendations2. Vivli, ClinicalStudyDataRequest and Engagezone data-sharing platforms were used to access and analyse the individual studies. Patients were categorized based on symptom duration into early or established disease, requiring ≥ 20% of patients in each category for eligibility. The primary outcome was ASAS40 response, with secondary outcomes including ASAS and ASDAS outcomes, at the time of the primary endpoint. For each trial, treatment effects of b/tsDMARDs vs PBO were assessed through relative risks (RR) and RR ratios (RRR), defined according to the different symptom duration thresholds. Subsequently, a meta-analysis was conducted across all included RCTs, using random effects.
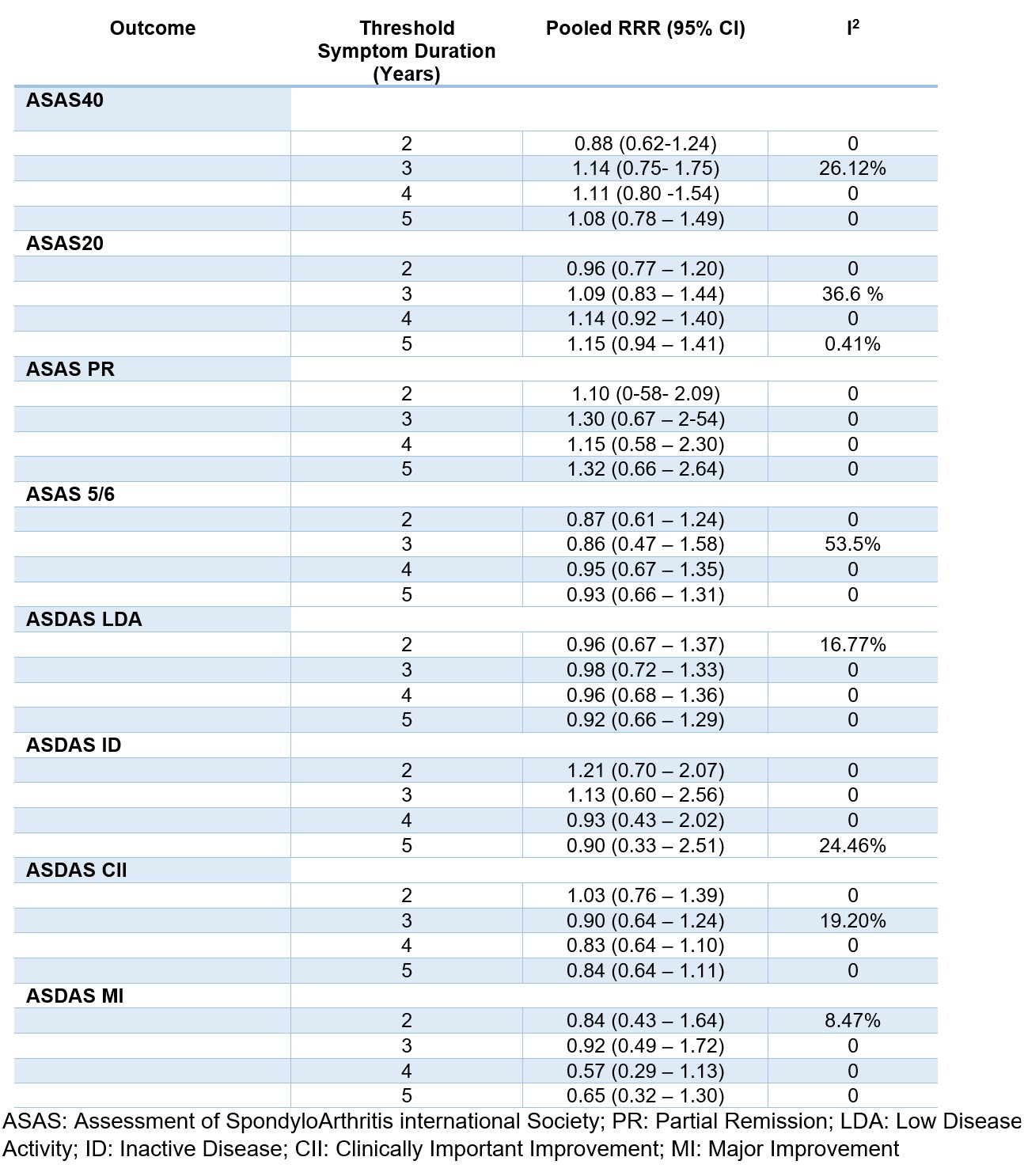
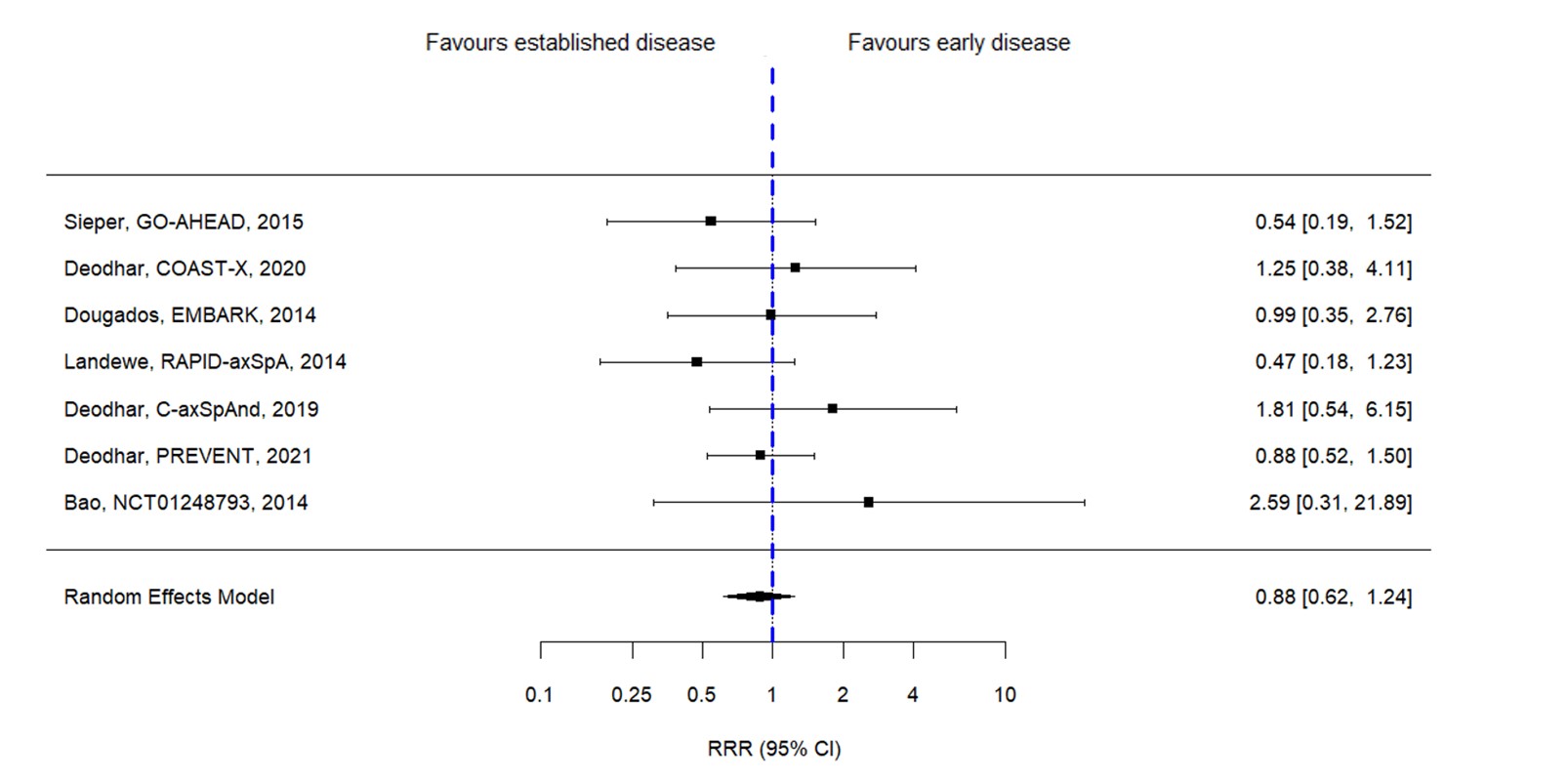
Results: In total 11 RCTs fulfilled the eligibility criteria and could be included, involving 3,272 patients with axSpA; 7 of these studies were eligible for the analysis of the primary endpoint for having ASAS40 response available. Among the included studies there were four studies with golimumab, two with certolizumab and secukinumab each, and one with adalimumab, etanercept and ixekizumab each- no studies with tsDMARDs were included. Mean (SD) age of patients was 37.9 (11.6) years, mean symptom duration 9.3 (9.2) years and 59% were male. Figure 1 shows the pooled RRR of bDMARDs vs PBO in early vs established disease for different outcomes and according to different symptom duration thresholds. Only 1 study was eligible for the 1-year cutoff and therefore pooled outcomes could not be assessed. With the 2-year threshold, the RR for the ASAS40 at the timing of the primary endpoint (weeks 12-16) with bDMARD vs PBO was not different in patients with early disease (RR 2.04 [1.48–2.84]) as compared with established disease (RR 2.28 [1.65–3.15]). None of the individual studies yielded a significant RRR for ASAS40 for early versus established disease (Figure 2). The pooled ASAS40 RRR for the 2-year threshold was 0.88 [0.62-1.24]). With the 3-year threshold, the RRR for ASAS40 was 1.14 [0.75-1.75]), with 4-years 1.11 [0.80-1.54]) and with 5-years, 1.08 [0.78-1.49]). None of the remaining outcomes yielded a significant RRR for early vs established disease according to different thresholds.
Conclusion: In this meta-analysis of RCTs, no significant difference has been found in the effect of bDMARDs vs PBO in early compared to established axSpA, defined according to several thresholds of symptom duration (between 2 and 5 years). Treating patients earlier or later in the disease course translates into the achievement of comparable short-term clinical outcomes.
1. Navarro-Compán V et al. Ann Rheum Dis. 2023;ard-2023-224232.
2. Webers C et al. Ann Rheum Dis.2023;82(1):130-141.
To cite this abstract in AMA style:
Benavent D, Navarro Compán V, Capelusnik D, Ramiro S. Efficacy of bDMARDs in Early versus Established Disease in Axial Spondyloarthritis: A Meta-Analysis of Randomized Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-bdmards-in-early-versus-established-disease-in-axial-spondyloarthritis-a-meta-analysis-of-randomized-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-bdmards-in-early-versus-established-disease-in-axial-spondyloarthritis-a-meta-analysis-of-randomized-trials/