Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Prior exposure to biologic (b)DMARD therapy of patients (pts) with AS may influence treatment response.1-3 Tofacitinib is an oral JAK inhibitor for the treatment of AS. The impact of prior treatment on tofacitinib efficacy and safety in pts with AS was evaluated.
Methods: Data from a placebo (PBO)-controlled, double‑blind study of pts with active AS (NCT03502616)4 were analyzed. Pts received tofacitinib 5 mg twice daily (BID) or PBO for 16 weeks; after Week (W)16, all pts received open-label tofacitinib 5 mg BID to W48 and were analyzed by prior treatment subgroup: bDMARD‑naïve and TNF inhibitor (TNFi)‑inadequate responders (IR), including bDMARD‑experienced (non‑IR). Efficacy and pt-reported outcomes assessed to W16 (PBO controlled) and W48 (open label): Assessment in SpondyloArthritis international Society (ASAS)20/40 and LS mean change from baseline (Δ) in AS‑Disease Activity Score-CRP (ASDAS-CRP), high-sensitivity (hs)CRP (mg/dL), BASFI, BASMI, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Short Form‑36 Physical Component Summary (SF-36v2 PCS), AS Quality of Life (ASQoL), total back pain, pt global assessment (PtGA), and morning stiffness. Assessment of ASAS20/40 at W16 was pre‑specified; all other analyses were post hoc. Safety assessed throughout.
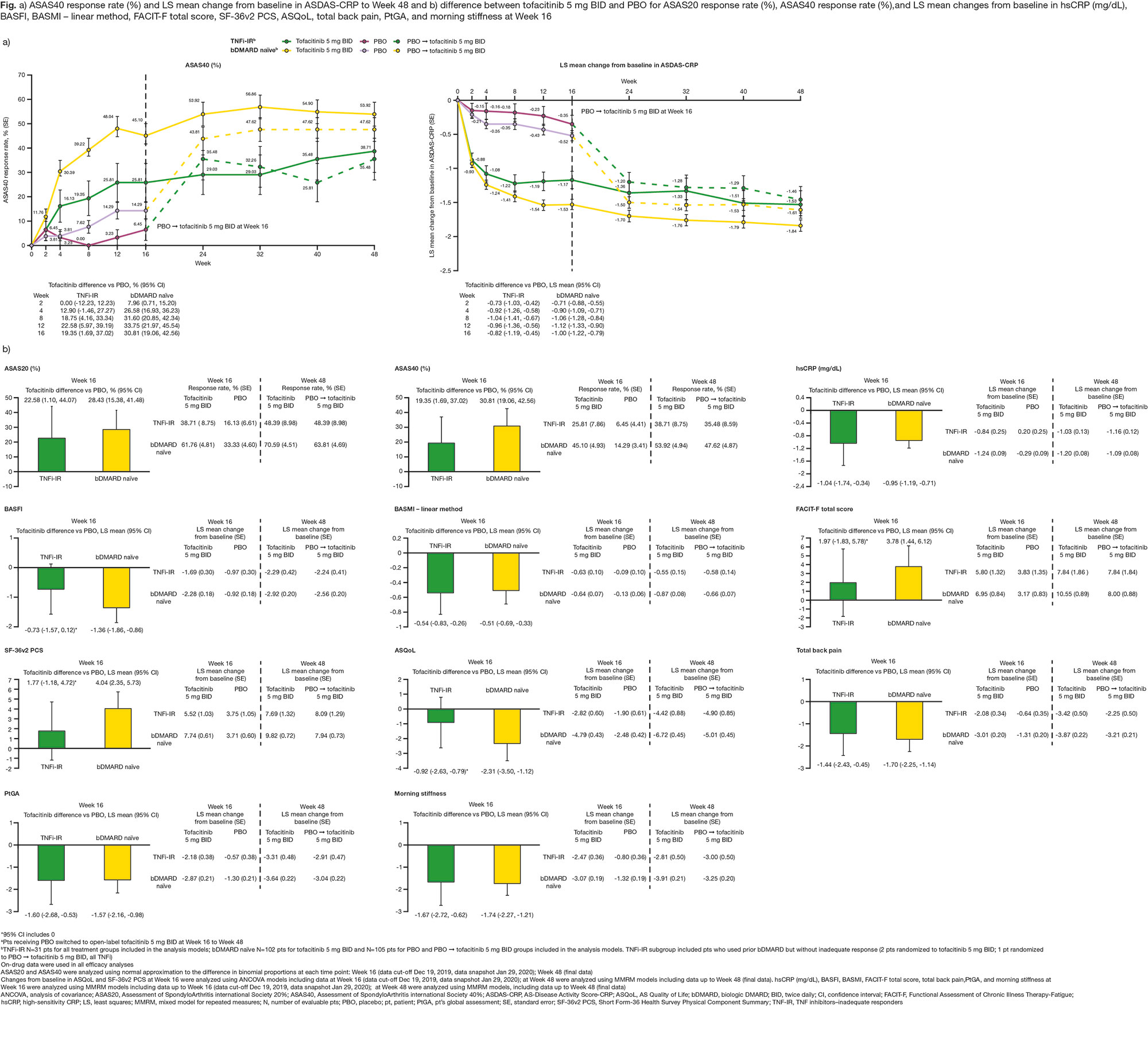
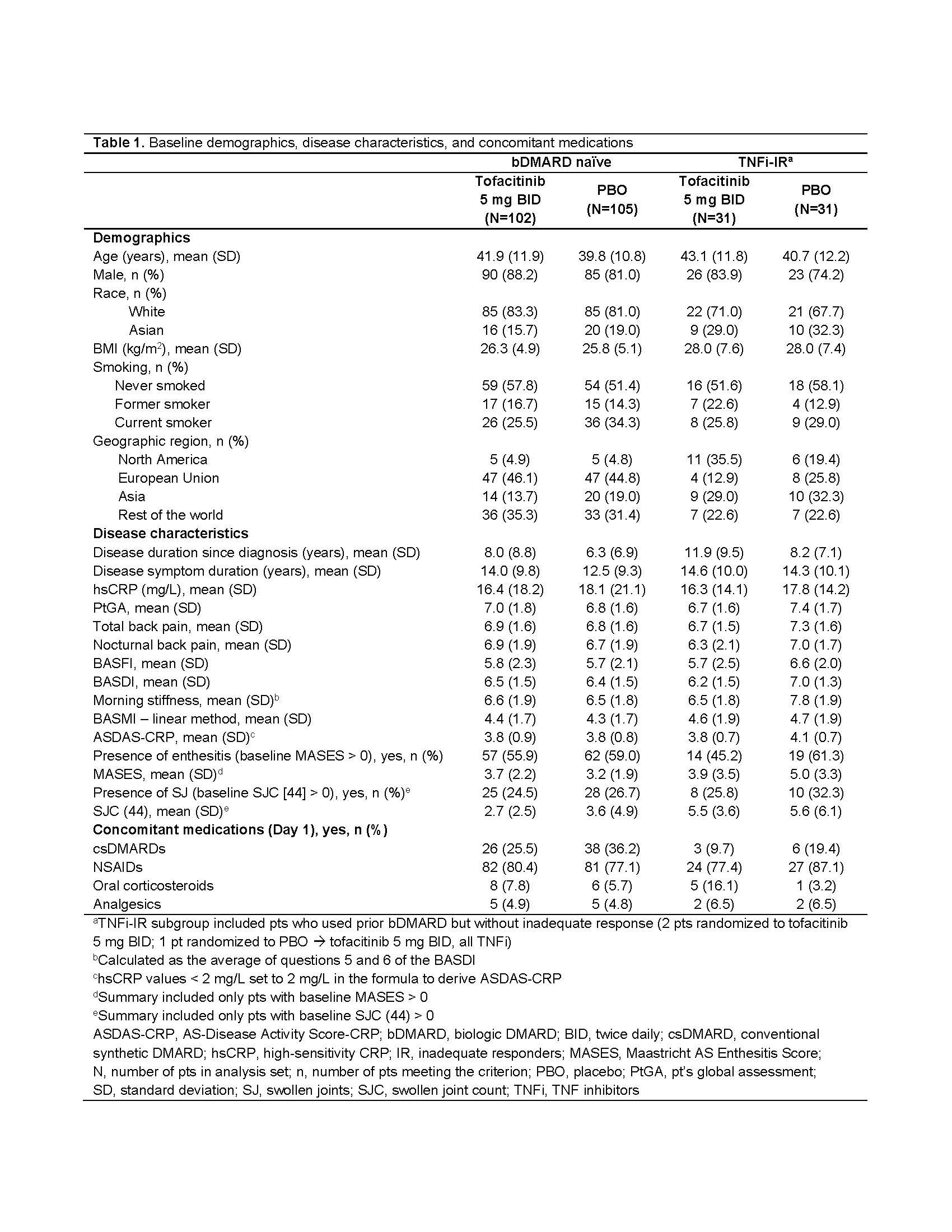
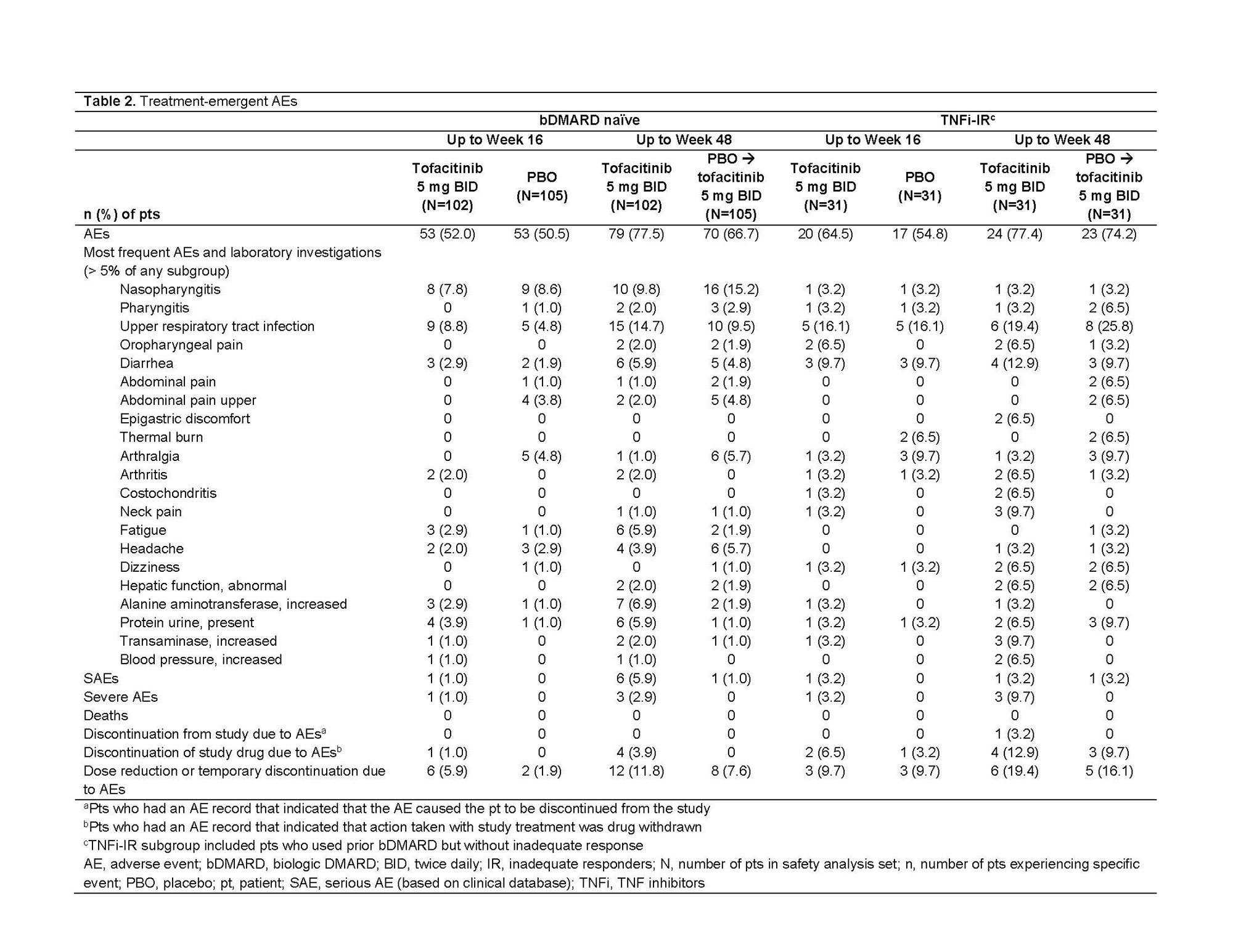
Results: This analysis included 269 randomized and treated pts: 207 (77%) bDMARD‑naïve and 62 (23%) TNFi-IR (incl. 3 bDMARD-experienced [non‑IR] pts, all TNFi). At baseline, TNFi‑IR had higher BMI, longer disease duration, and lower rates of conventional synthetic DMARD use vs bDMARD‑naïve pts; a greater % of TNFi-IR were from North America/Asia vs European Union/rest of world (Table 1). In TNFi-IR pts, baseline disease activity differed between treatment groups. At W16, for ASAS40 response and ΔASDAS-CRP, tofacitinib 5 mg BID efficacy was greater than PBO for bDMARD-naïve and TNFi-IR pts; efficacy was sustained up to W48 (Fig a). At W16, difference from PBO (95% CI) for tofacitinib 5 mg BID was similar for bDMARD-naïve and TNFi-IR pts across endpoints including ASAS20/40 response, Δ in hsCRP, BASFI, BASMI, FACIT‑F, SF‑36v2 PCS, ASQoL, total back pain, PtGA, and morning stiffness (Fig b). The % of pts with adverse events (AEs) was similar for TNFi‑IR vs bDMARD‑naïve pts for tofacitinib and PBO; no deaths were reported. The % of pts who discontinued or dose reduced/temporarily discontinued tofacitinib due to AE was numerically higher for TNFi‑IR vs bDMARD-naïve pts for tofacitinib and PBO (Table 2).
Conclusion: Tofacitinib efficacy was greater vs PBO at W16 for bDMARD‑naïve and TNFi-IR pts, with differences from PBO generally consistent between both groups and sustained up to W48. More TNFi-IR pts experienced discontinuations, dose reduction, or temporary discontinuation due to AE vs bDMARD-naïve pts. This analysis was limited by the small sample size overall and differences in sample size between subgroups.
1. Dougados et al. Ann Rheum Dis 2020; 79: 176-85
2. Deodhar et al. Arthritis Rheum 2019; 71: 599-611
3. Navarro-Compán et al. RMD Open 2017; 3: e000524
4. Deodhar et al. Ann Rheum Dis 2021; 80: 1004-13
Study sponsored by Pfizer. Medical writing support provided by L Rodgers, CMC Connect, and funded by Pfizer.
To cite this abstract in AMA style:
Deodhar A, Marzo-Ortega H, Wu J, Wang C, Dina O, Kanik K, Fallon L, Gensler L. Efficacy and Safety of Tofacitinib in Patients with Ankylosing Spondylitis by Prior bDMARD Treatment: Analysis of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-ankylosing-spondylitis-by-prior-bdmard-treatment-analysis-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-ankylosing-spondylitis-by-prior-bdmard-treatment-analysis-of-a-phase-3-trial/