Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases III
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: To investigate the efficacy and safety of therapies currently in use and under evaluation for systemic Juvenile Idiopathic Arthritis (sJIA) and Adult-Onset Still’s disease (AOSD).
Methods: A systematic review (SR) was performed. Medline, Embase, and the Cochrane Library databases were searched up to October 2022 for clinical trials (randomised, RCT, and quasi-controlled, CCT), longitudinal observational studies (retrospective, LOR, and prospective, LOP) and SRs published after 2013. The research question was formulated according to the PICO format. Population: sJIA (fulfilling ILAR criteria) or AOSD (fulfilling either Yamaguchi’s and/or Fautrel’s criteria) patients;
Intervention: any pharmacological treatment (in use or under evaluation for sJIA/AOSD); Comparator: any other active drug or placebo; Outcomes: any relevant efficacy and safety outcome. The risk of bias (RoB) of the included clinical studies was assessed with the Cochrane RoB tool, while AMSTAR-2 was used to critically assess SRs.
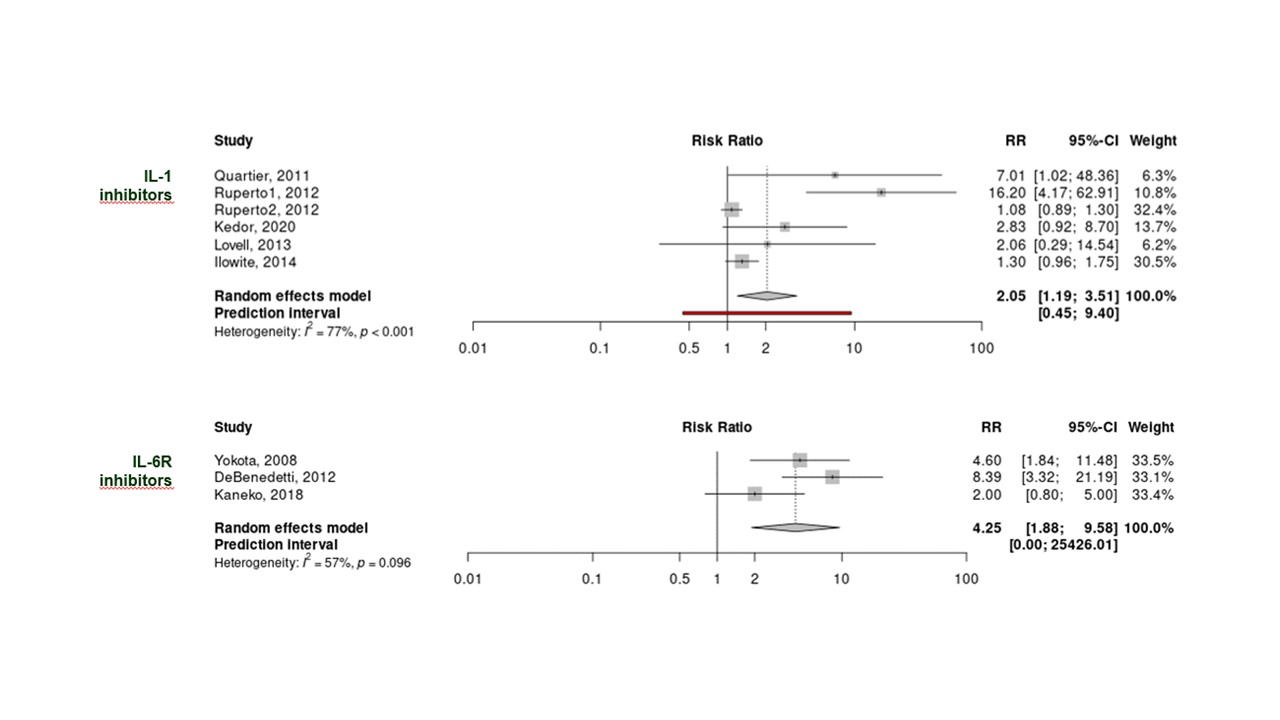
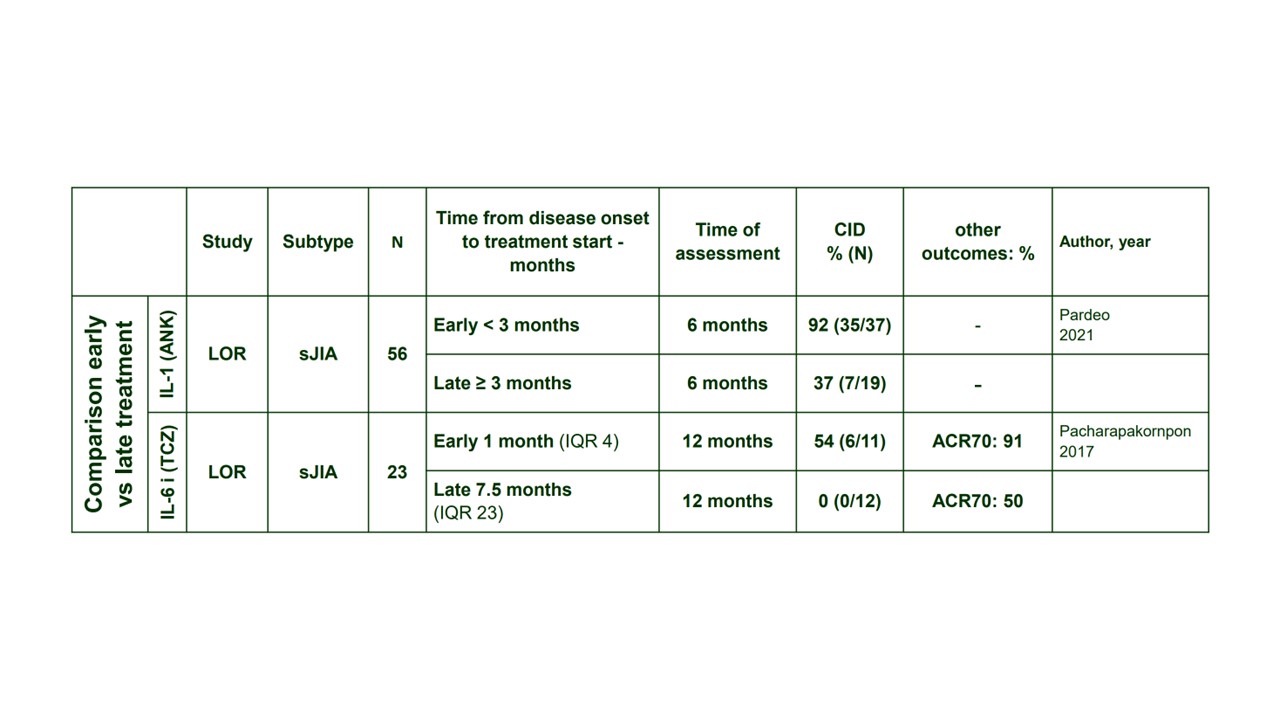
Results: Of 3,941 records, 115 full texts were finally included, representing 25 RCTs, 10 SRs published after 2013 and 80 LOR or LOP studies. Studies on glucocorticoids (GCs) and conventional synthetic DMARDs were mainly observational and displayed high RoB for the majority of them. Biologic DMARD (bDMARD) targeting IL-1 (IL-1i) or IL-6R (IL-6Ri) were the drugs with the highest level of evidence (6 clinical trials for IL-1i and 3 for IL-6Ri). A meta-analysis was conducted accordingly, using adapted ACR50 response as the outcome measure, and identified significant effect of both bDMARDs with RR 2.05 (95%CI 1.19, 3.51) and 4.25 (95%CI 1.88, 9.58) for IL1i and IL-6Ri respectively (Figure). Additionally, 2 retrospective longitudinal studies have demonstrated that the benefit of early bDMARD introduction, i.e. < 3 months, was associated with higher rates of clinically inactive disease (Table). This is consistent with data from other non-comparative studies in which clinically inactive disease (CID) achievement rates are between 59 to 100% when IL-1 or IL-6 inhibitors are initiated within 3 to 6 months, and 23 to 32% when they are started later on. Data on tsDMARDs (JAK-inhibitors), with promising findings, were limited to a few small case series.
Conclusion: Besides glucocorticoids, IL-1 and IL-6 inhibitors yielded the highest level of evidence for the treatment of sJIA and AOSD and their use early during the disease course was markedly efficacious.
To cite this abstract in AMA style:
Bindoli S, De Matteis A, MITROVIC S, Fautrel B, Carmona L, De Benedetti F, Task Force Member O. Efficacy and Safety of Therapeutic Interventions for the Treatment of Still’s Disease: A Systematic Review and Meta-analysis Informing the EULAR/PReS Recommendations for the Diagnosis and Management of Systemic Juvenile Idiopathic Arthritis and Adult-Onset Still’s Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-therapeutic-interventions-for-the-treatment-of-stills-disease-a-systematic-review-and-meta-analysis-informing-the-eular-pres-recommendations-for-the-diagnosis-and-mana/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-therapeutic-interventions-for-the-treatment-of-stills-disease-a-systematic-review-and-meta-analysis-informing-the-eular-pres-recommendations-for-the-diagnosis-and-mana/