Session Information
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: To describe the efficacy and safety of the SARS-CoV-2 vaccine in a series of patients with rheumatoid arthritis (RA).
Methods: Retrospective observational study of RA patients, fulfilling the ACR/EULAR 2010 classification criteria, vaccinated against the SARS-CoV-2 virus from December 2020 to October 2021, who had post-vaccination serology and subsequent follow-up for a minimum of 6 months in a university hospital. The efficacy and effectiveness of the vaccine was evaluated by analyzing the serological response (anti-SARS-CoV-2 IgG antibodies) and the incidence of post-vaccination SARS-CoV-2 infection. Vaccine safety was investigated recording adverse events and RA flares. Logistic and linear multivariate regression analyzes adjusted for age, sex, and time elapsed from vaccination to serological determination were used to identify factors associated with vaccine response. Statistical analysis was carried out with Stata® and significance was set at p< 0.05.
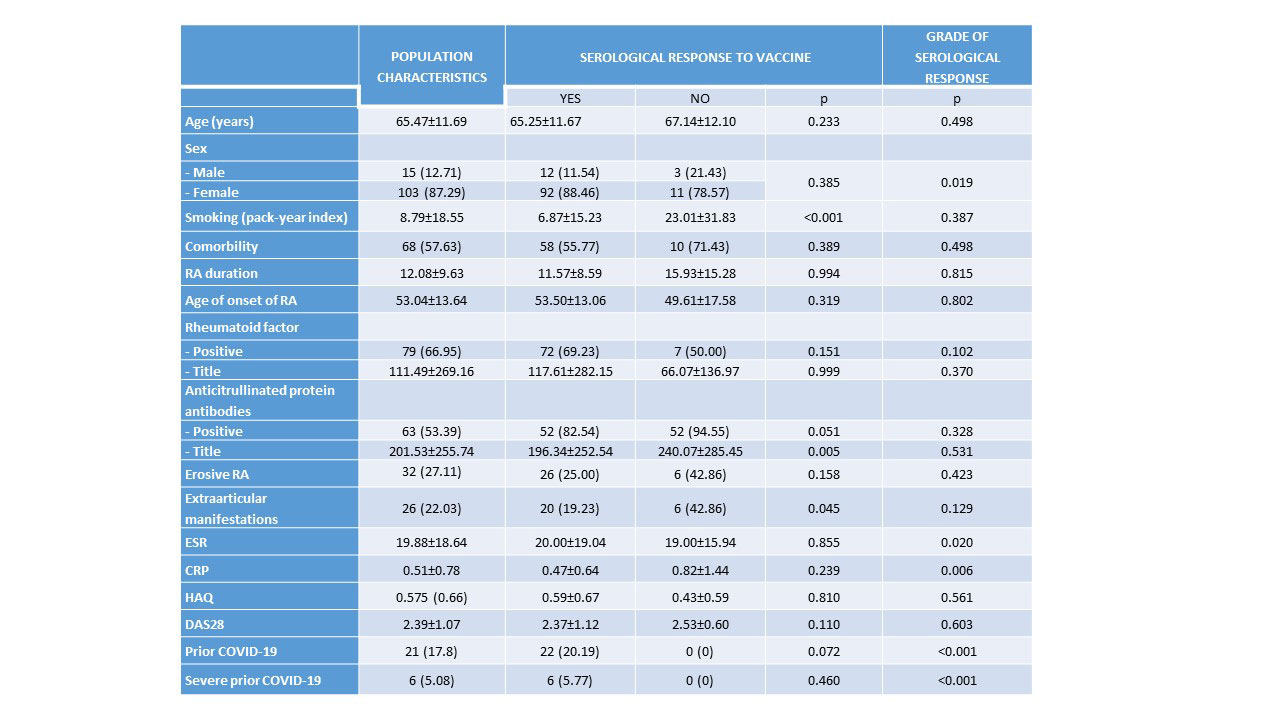
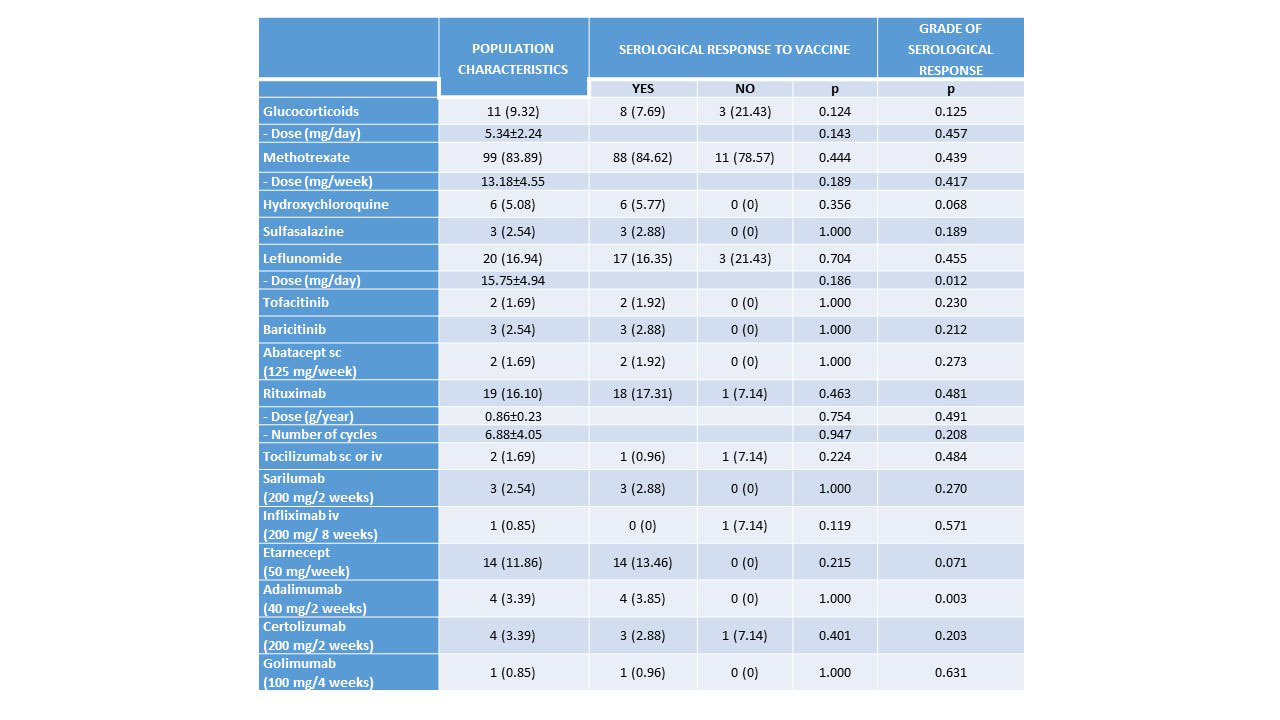
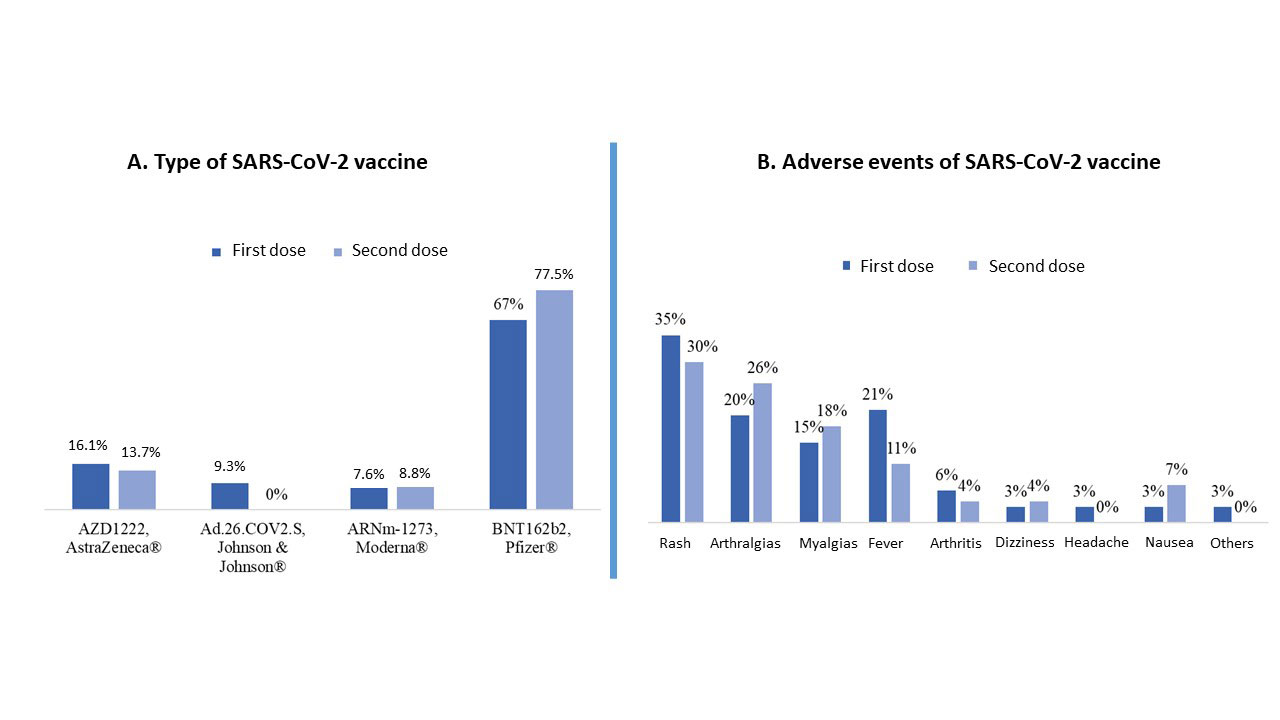
Results: We included 118 patients with RA (87.2% women, mean age 65.4±11.6 years, mean evolution 12.0±9.6 years) vaccinated against SARS-CoV-2 (95.76% received the complete vaccination schedule). Patient characteristics are shown in Table 1 and Table 2 and types of vaccines and schedule are shown in Image 1. Most patients (88.1%) developed humoral immunogenicity. Univariate analysis of factors associates with humoral response and grade of response to the vaccine are shown in Table 1 and Table 2. Multivariate analysis found that smoking (OR 0.96, 95%CI: 0.94-0.99, p=0.034) was the only variable significantly associated with humoral response to the vaccine. The degree of serological response was significantly related to younger age (coefficient -16.11, 95%CI: -31.84 to -0.39, p=0.045) and previous COVID-19 infection (coefficient 4.496, IC 95%: 3.604-5.388, p< 0.001), which had occurred in 17.8% (5% severe) and was associated with male gender (p=0.016). After vaccination, 18.6% presented SARS-CoV-2 infection, although mild. Vaccine adverse events (19.5%) were mostly mild and associated with age (OR 0.95, 95%CI: 0.91–0.99, p=0.042). RA flares were anecdotal (1.7%), being inversely related to age (OR 0.95, 95%CI: 0.91-0.99, p=0.042).
Conclusion: Our results, with a limited sample, suggest that the SARS-CoV-2 vaccine induces adequate humoral immunogenicity, with an acceptable safety profile in RA patients, reducing the severity of SARS-CoV-2 infection.
To cite this abstract in AMA style:
Vicente-Rabaneda E, Torres M, Uriarte M, Quiroga-Colina P, Gutiérrez A, Triguero A, Gutiérrez F, García N, Romero-Robles A, Cardeñoso L, Castañeda S. Efficacy and Safety of the SARS-CoV-2 Vaccine in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-the-sars-cov-2-vaccine-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-the-sars-cov-2-vaccine-in-patients-with-rheumatoid-arthritis/