Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: AP1189 is a novel, first-in-class, oral, biased melanocortin (MC)1 and MC3 receptor agonist in development for Rheumatoid Arthritis (RA) treatment. Through multiple actions including reduction in pro-inflammatory cells and cytokines and increased macrophage efferocytosis, it induces inflammation resolution in animal models. In a Phase 2a study in newly diagnosed MTX-naïve RA patients with high disease activity (CDAI >22), AP1189 100 mg+MTX showed a significant treatment effect compared to placebo+MTX treatment, with a significant reduction in CDAI and achieving an ACR20 response of 60.6 % at week 4. The purpose of the current randomized controlled trial was to evaluate 12 weeks treatment in MTX-naïve RA patients with high disease activity.
Methods: The EXPAND trial was a twelve-week, double-blind, placebo-controlled Phase 2b clinical trial that enrolled MTX-naïve patients presenting with CDAI > 22. The primary study objectives were to evaluate the efficacy, safety, and tolerability relative to placebo. The primary efficacy endpoint was defined as the proportion of patients reaching ACR20 at the completion of the 12-week treatment period. MTX therapy escalation regimen was at physician’s discretion and was initiated concurrently with AP1189/placebo. Participants were randomized to receive either 100 mg AP1189 or matched placebo tablets once daily in addition to MTX. Additional efficacy assessments included CDAI, DAS28-CRP and HAQ-DI. Glucocorticoid treatment was only permitted as rescue treatment after 4 weeks of study treatment.
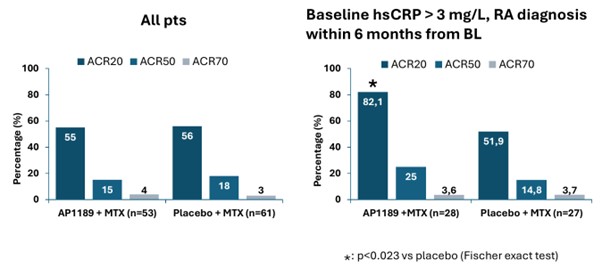
Results: Of 127 randomized patients, 114 completed the study. 87% female; mean age 56.5 years. Baseline CDAI (mean ± SD): Placebo+MTX 40.3 ± 10.1; AP1189+MTX 42.0 ± 12.2. 54% of the patients had hsCRP >3 mg/L. 22% of patients had > 6 months between RA diagnosis and initiation of study treatments. Efficacy evaluation on the iTT population could not identify any differences between AP1189+MTX and placebo+MTX treatment, with 56% of placebo and 55% of the AP1189 treated patients reaching ACR20 at week 12; similarly, there were no significant differences in the secondary efficacy outcomes. Adverse events (AEs) were equally distributed between the groups. AP1189 was generally well tolerated with no increased rate of infections, no signs of liver- or other abnormalities. However, three subjects on AP1189 discontinued treatment due to upper GI AEs. A post hoc analysis in patients who were newly diagnosed (RA diagnosis within 6 months of baseline) and who had elevated hsCRP identified an improved treatment response to AP1189 (n=28) relative to placebo (n=27) treatment – patients achieving ACR20, AP1189+MTX 82% vsplacebo+MTX: 52%; mean±SD reduction in DAS28-CRP: AP1189+MTX, 1.9±1.2 vs placebo+MTX 1.2±1.1; CDAI: AP1189+MTX 24.6±14.2 vs placebo+MTX 14.7±12.3; and HAQ-DI: AP1189+MTX 0.7±0.7 vs placebo+MTX: 0.3±0.7.
Conclusion: While there was no observed difference between AP1189 in combination with MTX and placebo+MTX in the study cohort as a whole, post hoc analyses in newly diagnosed RA patients with high disease activity including elevated hsCRP, together with safety data, suggest that AP1189 could be a promising treatment option in this patient group.
To cite this abstract in AMA style:
Bihlet A, Conaghan P, Boesen T, Jensen M, Mondragón A, Petersen E, Bak C, Jonassen T. Efficacy and Safety of the Biased Melanocortin Receptor Agonist AP1189/resomelagon in Combination with Methotrexate in DMARD-naïve Rheumatoid Arthritis Patients: The EXPAND Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-the-biased-melanocortin-receptor-agonist-ap1189-resomelagon-in-combination-with-methotrexate-in-dmard-naive-rheumatoid-arthritis-patients-the-expand-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-the-biased-melanocortin-receptor-agonist-ap1189-resomelagon-in-combination-with-methotrexate-in-dmard-naive-rheumatoid-arthritis-patients-the-expand-trial/