Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab has demonstrated efficacy and safety in patients with enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) categories of juvenile idiopathic arthritis (JIA) for up to 2 years.1 After completion of a 2-year primary study (JUNIPERA), a long-term extension (LTE) study was conducted to evaluate the continued efficacy and safety of secukinumab in patients with ERA and JPsA. Here we report the interim efficacy and safety results of the LTE study.
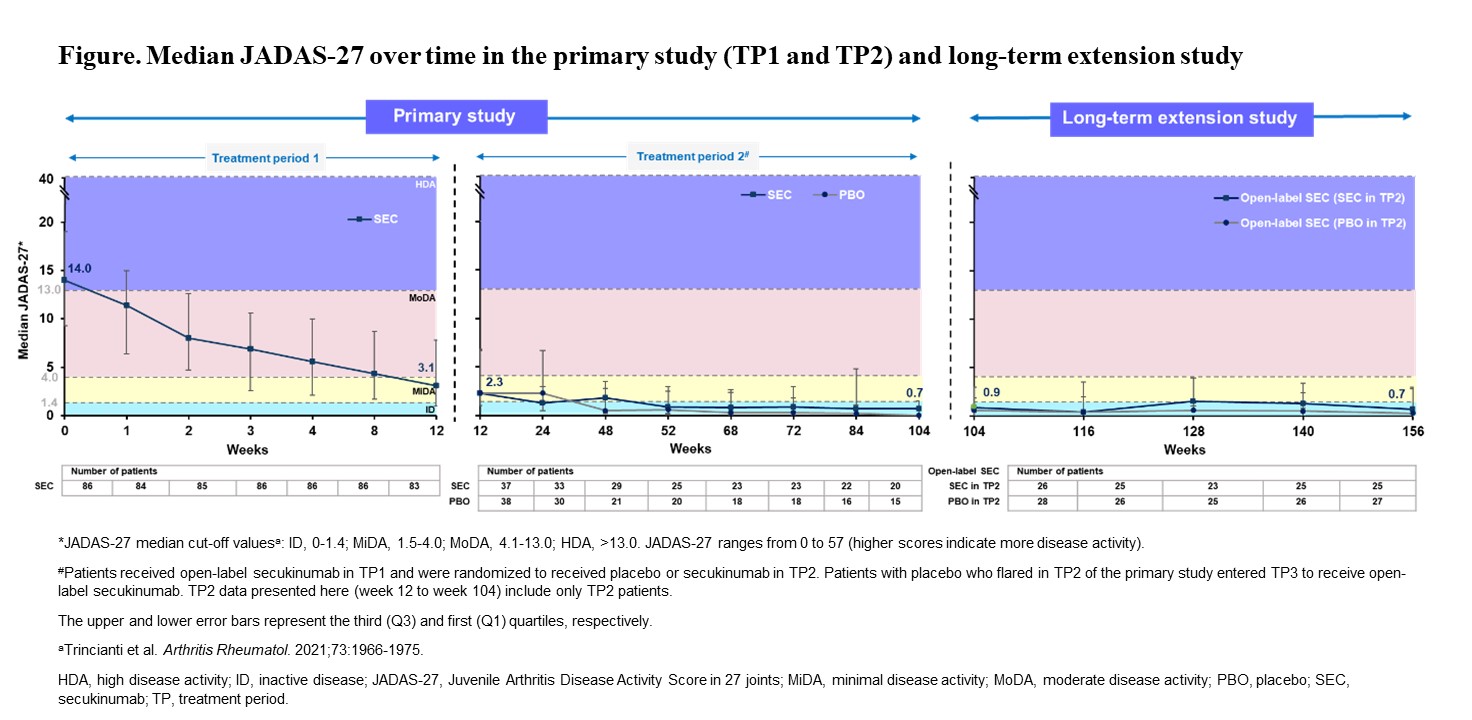
Methods: In the primary study, a total of 86 patients (2 to < 18 years of age) received secukinumab up to week 12 in the open-label period.1 JIA American College of Rheumatology (ACR)30 responders at week 12 (n=75) were subsequently randomized to secukinumab (n=37) or placebo (n=38) up to week 100 in study period 2. Those who flared after randomization (secukinumab, n=10; placebo, n=21) received open-label secukinumab in study period 3 up to week 100.1 A total of 55 of 61 patients who had completed the primary study consented to enter the LTE study, among which 54 patients received secukinumab (subcutaneous; 75/150 mg in patients < 50/≥50 kg) every 4 weeks up to 4 years. Patients whose signs and symptoms were not fully controlled, as judged by the investigator in the LTE study, could have dose escalation of their secukinumab dose from 75 mg to 150 mg or 150 mg to 300 mg. Median Juvenile Arthritis Disease Activity Scores (JADAS)-27 were presented up to week 156 for efficacy, and adverse events (AEs) and serious AEs were presented for the entire treatment period up to the cut-off date (02-Feb-2022).
Results: JADAS-27 in the primary study and in the LTE study are presented in the Figure. A total of 19 patients had dose escalation in the LTE study: 8 patients from 75 mg to 150 mg and 11 patients from 150 mg to 300 mg. The overall exposure-adjusted incidence rate per 100 patient-years (PY) of treatment-emergent AEs was 98.4 PY in the entire JIA population. The most commonly reported AEs were nasopharyngitis (n=9, 16.7%) and arthralgia (n=8, 14.8%). One major adverse cardiovascular event, not related to the study drug, and 2 cases of uveitis were reported. No cases of Crohn’s disease or deaths were reported, and no patient discontinued treatment due to an AE.
Conclusion: With secukinumab treatment, the JADAS-27 inactive disease status was sustained from week 104 to week 156 in patients with JIA who had completed the 2-year primary study and enrolled in the LTE study. Safety data were consistent with adult and pediatric indications, with no new or unexpected safety signals.
Reference:
1. Brunner HI, et al. Ann Rheum Dis. 2023;82(1):154-160.
To cite this abstract in AMA style:
Brunner H, Foeldvari I, Alexeeva E, Ayaz N, Schulert G, Ozen S, Popov A, Ramanan A, Scott C, Sozeri B, Zholobova E, Chakraborty S, Zhu X, Martin R, Whelan S, Kaur S, Pricop L, Lovell D, Martini A, Ruperto N. Efficacy and Safety of Secukinumab in Juvenile Idiopathic Arthritis: Interim Results from the Extension of the JUNIPERA Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-secukinumab-in-juvenile-idiopathic-arthritis-interim-results-from-the-extension-of-the-junipera-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-secukinumab-in-juvenile-idiopathic-arthritis-interim-results-from-the-extension-of-the-junipera-trial/