Session Information
Date: Monday, November 11, 2019
Title: 4M097: Systemic Sclerosis & Related Disorder – Clinical II: Cardiopulmonary Involvement (1830–1835)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: In the SENSCIS trial in patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD), nintedanib reduced the annual rate of decline in forced vital capacity (FVC) vs placebo (-52.4 vs -93.3 mL/year; difference 41.0 mL/year [95% CI 2.9, 79.0]; p=0.04), with adverse events that were consistent with the profile observed in patients with IPF. In many countries, mycophenolate is commonly used in the treatment of SSc-ILD. We analyzed the efficacy and safety of nintedanib in the SENSCIS trial by use of mycophenolate at baseline.
Methods: Subjects with SSc-ILD with ≥10% fibrosis of the lungs on HRCT were randomized to receive nintedanib 150 mg bid or placebo. Patients who had received stable therapy with mycophenolate for ≥6 months prior to randomization were eligible to participate. We analyzed lung function outcomes and adverse events over 52 weeks in subgroups of patients who were and were not taking mycophenolate at baseline.
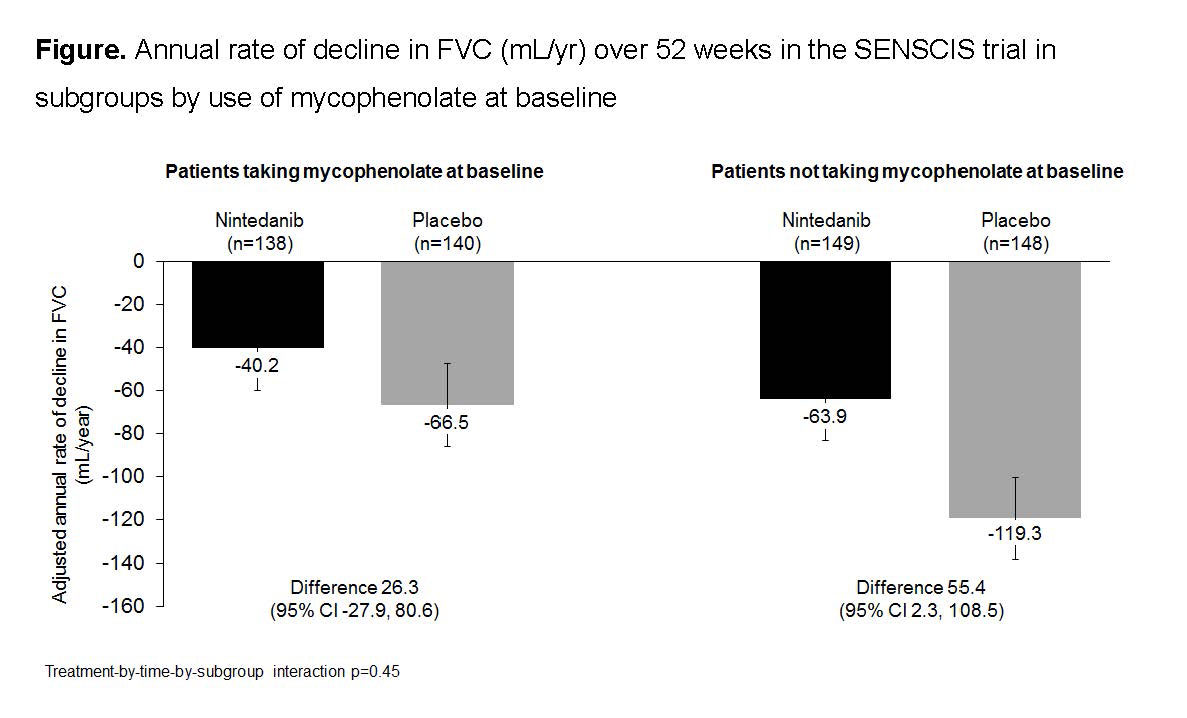
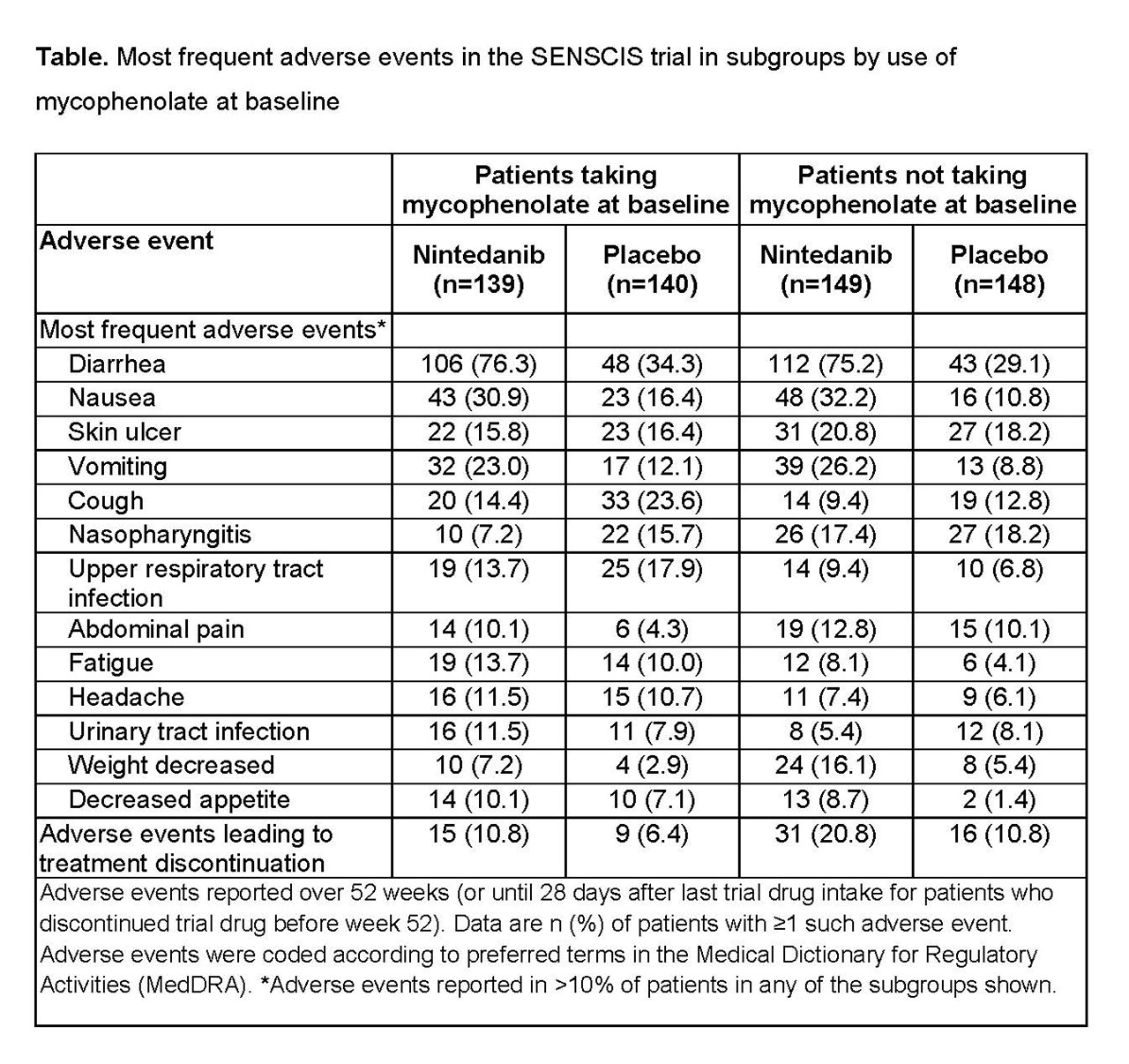
Results: In the nintedanib and placebo groups, respectively, 139 (48.3%) and 140 (48.6%) of patients were taking mycophenolate at baseline. In patients taking and not taking mycophenolate at baseline, respectively, mean (SD) FVC (mL) was 2539 (770) and 2463 (784) and FVC % predicted was 70.8 (16.0) and 74.2 (17.1). In patients who received placebo, the mean (SE) rate of decline in FVC over 52 weeks was -66.5 (19.3) mL/year in patients taking mycophenolate at baseline and -119.3 (19.0) mL/year in patients not taking mycophenolate at baseline. Nintedanib reduced the rate of FVC decline both in patients who were and were not taking mycophenolate at baseline. The treatment effect of nintedanib was numerically greater in patients who were not taking mycophenolate at baseline, but statistical testing did not indicate heterogeneity in the treatment effect between subgroups (p=0.45) (Figure). In post-hoc analyses, in the nintedanib and placebo groups, respectively, absolute declines in FVC >5% predicted were seen in 15.2% and 25.7% of patients taking mycophenolate at baseline (OR 0.52 [95% CI 0.29, 0.95]) and 25.5% and 31.1% of those not taking mycophenolate at baseline (OR 0.76 [0.46, 1.26]). The adverse event profile of nintedanib was similar irrespective of mycophenolate use at baseline (Table). The proportion of patients treated with nintedanib who had adverse events leading to discontinuation of trial drug was no higher in patients taking mycophenolate at baseline than in those who were not (Table).
Conclusion: In the SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in FVC both in patients who had not taken mycophenolate and in patients who had taken a stable dose of mycophenolate for ≥6 months prior to randomization. The treatment effect of nintedanib was numerically greater in patients who were not taking mycophenolate at baseline. Careful interpretation of the data in the subgroups by use of mycophenolate is warranted as patients were not randomized by use of mycophenolate and the patients using mycophenolate at baseline had tolerated it for ≥6 months prior to entering the trial. The adverse event profile of nintedanib was similar irrespective of mycophenolate use.
To cite this abstract in AMA style:
Highland K, Distler O, Kuwana M, Allanore Y, Assassi S, Azuma A, Bourdin A, Denton C, Distler J, Hoffmann-Vold A, Khanna D, Mayes M, Raghu G, Vonk M, Gahlemann M, Girard M, Stowasser S, Zoz D, Fischer A, Maher T. Efficacy and Safety of Nintedanib in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease by Use of Mycophenolate at Baseline: Subgroup Analysis of the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-by-use-of-mycophenolate-at-baseline-subgroup-analysis-of-the-senscis-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-by-use-of-mycophenolate-at-baseline-subgroup-analysis-of-the-senscis-trial/