Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In the SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in forced vital capacity (FVC) vs placebo. Patients with SSc-ILD frequently have comorbidities that add to their functional impairment and complicate their care. We investigated the efficacy and safety of nintedanib in subgroups based on comorbidity burden.
Methods: The SENSCIS trial enrolled patients with SSc-ILD with first non-Raynaud symptom in the last ≤7 years and an extent of fibrotic ILD on HRCT ≥10%. Patients with clinically significant pulmonary hypertension were excluded. Comorbidities at baseline were counted in categories based on organ group. In the Charlson Comorbidity Index (CCI), ages of 50–59 years, 60–69 years, 70–79 years and ≥80 years are assigned 1, 2, 3 and 4 points, respectively. The presence and severity of specific comorbidities are scored 1, 2, 3 or 6 points each. Patients with certain comorbidities used to calculate the CCI (e.g. metastatic solid tumor, AIDS) were not eligible for inclusion in the trial. SSc was not counted as a comorbidity. We investigated the rate of decline in FVC (mL/year) and adverse events over 52 weeks in subgroups with ≤2 vs >2 comorbidities and CCI score ≤1 vs >1 at baseline.
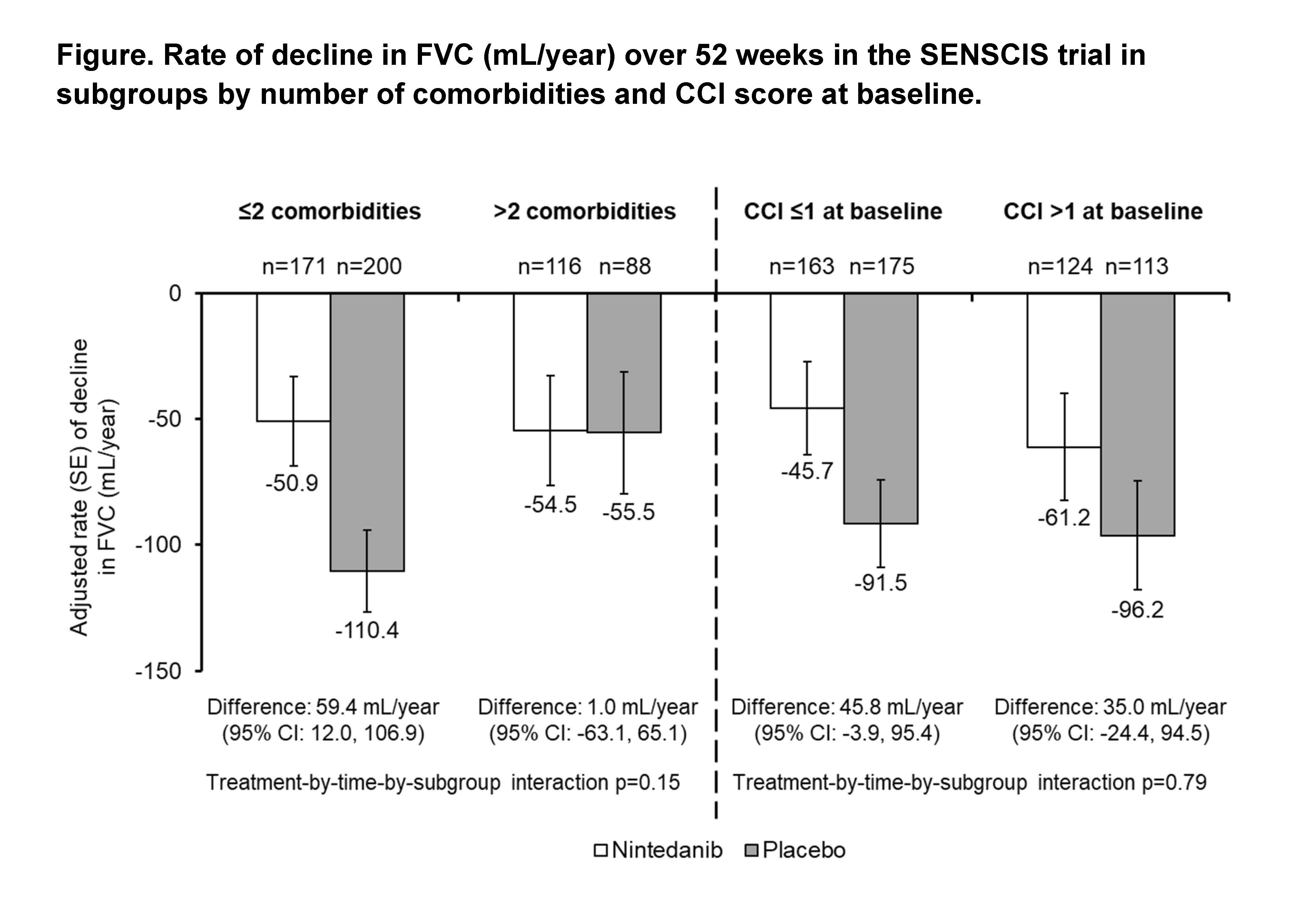
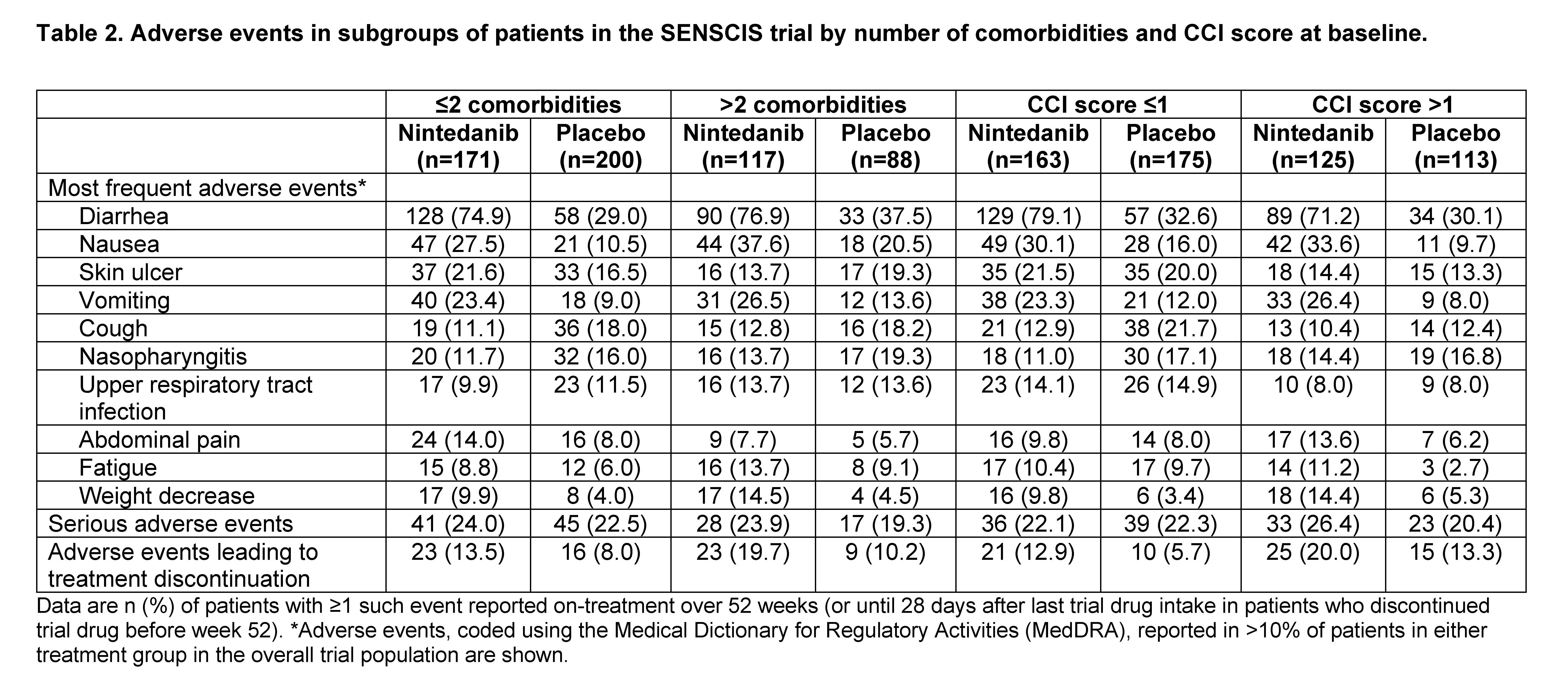
Results: Among 576 patients treated in the SENSCIS trial, 205 (35.6%) had >2 comorbidities and 238 (41.3%) had a CCI score >1 at baseline. At baseline, compared with patients with ≤2 comorbidities, those with >2 comorbidities had a higher mean age, FVC % predicted, and St George’s Respiratory Questionnaire total score, a greater proportion were female, and a smaller proportion had diffuse cutaneous SSc (dcSSc) (Table 1). Compared with patients with a CCI score ≤1, those with a CCI score >1 had a higher mean age and FVC % predicted and a smaller proportion had dcSSc (Table 1). In the placebo group, the rate of decline in FVC over 52 weeks was numerically greater in patients with ≤2 than >2 comorbidities, but similar between patients with CCI score ≤1 and >1. The effect of nintedanib versus placebo on reducing the rate of FVC decline was numerically greater in patients with ≤2 than >2 comorbidities and similar between patients with CCI score ≤1 and >1, but no heterogeneity in the treatment effect was detected (interaction p-values 0.15 and 0.79, respectively) (Figure). The adverse event profile of nintedanib was generally similar across the subgroups (Table 2). Adverse events leading to discontinuation were more frequent in patients treated with nintedanib than placebo and, in both treatment groups, were more frequent in patients with higher comorbidity burden.
Conclusion: In the SENSCIS trial in patients with SSc-ILD, patients with greater comorbidity burden had higher FVC at baseline. The rate of decline in FVC over 52 weeks in the placebo group, and the effect of nintedanib on the rate of FVC decline, were numerically greater in patients with ≤2 than >2 comorbidities, but no statistically significant heterogeneity was detected in the effect of nintedanib between the subgroups. Treatment discontinuation due to adverse events was more common in patients with greater comorbidity burden. Proactive management of adverse events is important to help patients stay on antifibrotic therapy.
To cite this abstract in AMA style:
Highland K, Moua T, Aringer M, Ogura T, Miede C, Alves M, Steen V. Efficacy and Safety of Nintedanib in Patients with Systemic Sclerosis-Associated ILD (SSc-ILD) and Differing Comorbidity Burden: Subgroup Analyses of the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-nintedanib-in-patients-with-systemic-sclerosis-associated-ild-ssc-ild-and-differing-comorbidity-burden-subgroup-analyses-of-the-senscis-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-nintedanib-in-patients-with-systemic-sclerosis-associated-ild-ssc-ild-and-differing-comorbidity-burden-subgroup-analyses-of-the-senscis-trial/