Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II: Models and Mechanisms (1662–1667)
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: Nerandomilast is a preferential inhibitor of phosphodiesterase 4B with antifibrotic and immunomodulatory properties. The Phase III FIBRONEER-ILD trial in patients with progressive pulmonary fibrosis (PPF) showed that nerandomilast significantly reduced the decline in forced vital capacity (FVC) and had an acceptable safety profile. We explored the efficacy and safety of nerandomilast in the subgroup of patients with autoimmune disease-related interstitial lung diseases (ILDs) (autoimmune ILDs) in the FIBRONEER-ILD trial.
Methods: Patients with PPF (excluding idiopathic pulmonary fibrosis) were randomized 1:1:1 to receive nerandomilast 9 mg bid, nerandomilast 18 mg bid, or placebo. PPF was defined using the same criteria as in the INBUILD trial (Flaherty KR et al. N Engl J Med 2019;381:1718-27). Patients taking nintedanib (at a stable dose for ≥12 weeks) or not taking nintedanib (for ≥8 weeks) were eligible to participate. Cyclophosphamide, tocilizumab, mycophenolate, or rituximab were not permitted at enrolment but could be initiated after 6 months to manage worsening systemic disease. Prednisone >15 mg/day (or equivalent) was not permitted at enrolment but could be prescribed during the trial for acute exacerbation of ILD or after 6 months to manage worsening systemic disease. In the subgroup with autoimmune ILDs, we evaluated absolute change from baseline in FVC (mL) at week 52 and adverse events up to week 52. Analyses were pre-specified.
Results: Among 1176 treated patients, 325 (27.6%) had autoimmune ILDs (100 placebo, 112 nerandomilast 9 mg bid, 113 nerandomilast 18 mg bid). At baseline, among patients with autoimmune ILDs, 212 (65.2%) were female, mean (SD) age was 63.4 (11.2) years, FVC was 71.5 (15.0) % predicted, diffusing capacity for carbon monoxide (DLco) was 51.5 (16.8) % predicted; 111 (34.2%) patients were taking nintedanib. The most frequent autoimmune disease diagnoses were rheumatoid arthritis (118 patients [36.3%]), systemic sclerosis (75 [23.1%]), and mixed connective tissue disease (47 [14.5%]). Among patients with autoimmune ILDs, adjusted mean changes in FVC (mL) at week 52 were -107.1 (95% CI: -156.1, -58.0) in the placebo group, -61.2 (-106.9, -15.5) in the nerandomilast 9 mg bid group (difference vs placebo: 45.9 [95% CI: -20.8, 112.6]), and -64.9 (-111.0, -18.7) in the nerandomilast 18 mg bid group (difference vs placebo: 42.2 [-24.9, 109.3]) (Figure). The most frequent adverse event was diarrhea (Table). Adverse events leading to treatment discontinuation were similar across treatment groups.
Conclusion: In the FIBRONEER-ILD trial, the efficacy of nerandomilast on slowing decline in FVC in patients with autoimmune ILDs was consistent with that observed in the overall trial population. Nerandomilast had an acceptable safety and tolerability profile.
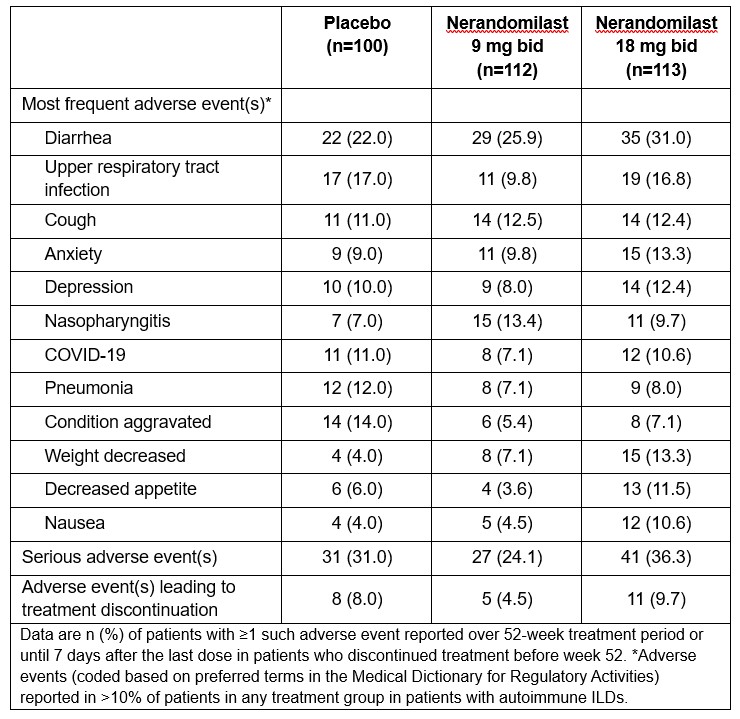 Figure. Change from baseline in FVC (mL) at week 52 among patients with autoimmune ILDs in the FIBRONEER-ILD trial.
Figure. Change from baseline in FVC (mL) at week 52 among patients with autoimmune ILDs in the FIBRONEER-ILD trial.
.jpg) Table. Adverse events up to week 52 in patients with autoimmune ILDs in the FIBRONEER-ILD trial.
Table. Adverse events up to week 52 in patients with autoimmune ILDs in the FIBRONEER-ILD trial.
To cite this abstract in AMA style:
Hoffmann-Vold A, Assassi S, Cottin V, Kreuter M, Valenzuela C, Wijsenbeek M, Gu H, Ritter I, Stowasser S, Weimann G, Maher T. Efficacy and Safety of Nerandomilast in Patients with Autoimmune Disease-Related Progressive Pulmonary Fibrosis: Subgroup Analysis of the FIBRONEER-ILD trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-nerandomilast-in-patients-with-autoimmune-disease-related-progressive-pulmonary-fibrosis-subgroup-analysis-of-the-fibroneer-ild-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-nerandomilast-in-patients-with-autoimmune-disease-related-progressive-pulmonary-fibrosis-subgroup-analysis-of-the-fibroneer-ild-trial/
