Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Dermatomyositis (DM) is a rare chronic systemic autoimmune disease with characteristic skin rash and progressive proximal muscle weakness. Intravenous immunoglobulin (IVIg) has long been used as adjuvant treatment for DM, but evidence from large randomized clinical trials supporting the efficacy and safety of IVIg in DM is limited.
The ProDERM study aimed to evaluate the efficacy and safety/tolerability of IVIg in DM patients in a double-blind, randomized, placebo-controlled, international multi-centre, phase III clinical trial.
Methods: The trial consisted of 2 periods of 16 and 24 weeks each. In the 1st double-blind, placebo-controlled period, adult patients with definite or probable DM (according to Bohan and Peter criteria) were randomized 1:1 to either high dose IVIg (2g/kg every 4 weeks) or placebo. To be included, subjects must have active disease with a manual muscle testing-8 (MMT-8) score < 142/150. Patients were switched to the alternate treatment arm if they showed clinical worsening (defined according to Oddis et al, 2013 – with slight adaptation) between week 8 and week 16. After week 16, all patients on placebo and patients without clinical worsening while on IVIg treatment entered the open label extension period, receiving 2 g/kg IVIg infusions every 4 weeks for 24 weeks. Primary endpoint was the proportion of responders in the IVIg vs. placebo arm at week 16, where response was defined per 2016 ACR/EULAR Myositis response criteria of at least minimal improvement [Total Improvement Score (TIS) ≥ 20 points)] and without clinical worsening at 2 consecutive visits up to week 16.
Results: A total of 95 adult DM patients (mean age: 53 years; 75% females; 92% Caucasian) were enrolled, with 47 and 48 randomized to IVIg and placebo, respectively. Baseline clinical characteristics were balanced between the 2 arms. At week 16, the proportion of responders was significantly higher in the IVIg group (37/47; 78.7%) as compared to the placebo group (21/48; 43.8%; p-value 0.0008) thus meeting the primary endpoint. Only 3 patients worsened in placebo group and none in IVIg group at or before 16 weeks. During the first period time to response was significantly shorter with IVIg (median of 35 days vs. 115 days for placebo; Fig 1).
The difference in response rate was even more marked for at least moderate improvement (TIS ≥ 40): 68.1% in the IVIg arm as compared to 22.9% in the placebo arm (p < 0.0001).
The mean TIS (SD) was significantly higher in IVIg group [48.4 (24.4)] than in placebo arm [21.6 (20.2)] at week 16 (Fig 2).
The proportion of responders as well as mean TIS at the end of the open-label extension period (week 16 to week 40) was similar in both treatment groups, showing that patients randomized to placebo in the first period improved after switching to IVIg in the extension period (Fig 1,2). Other secondary end points [e.g. MMT-8 and Cutaneous DM Disease Activity Area and Severity index (CDASI)], were also met, further supporting efficacy of IVIg.
The safety and tolerability profile of IVIg was consistent with previously reported safety outcomes.
Conclusion: This is the first large international phase III randomized, placebo-controlled trial demonstrating the efficacy and safety of IVIg as a treatment for patients with DM.
 Fig 1: Time to Response (at Least Minimal Improvement; TIS ≥ 20) in both treatment arms
Fig 1: Time to Response (at Least Minimal Improvement; TIS ≥ 20) in both treatment arms
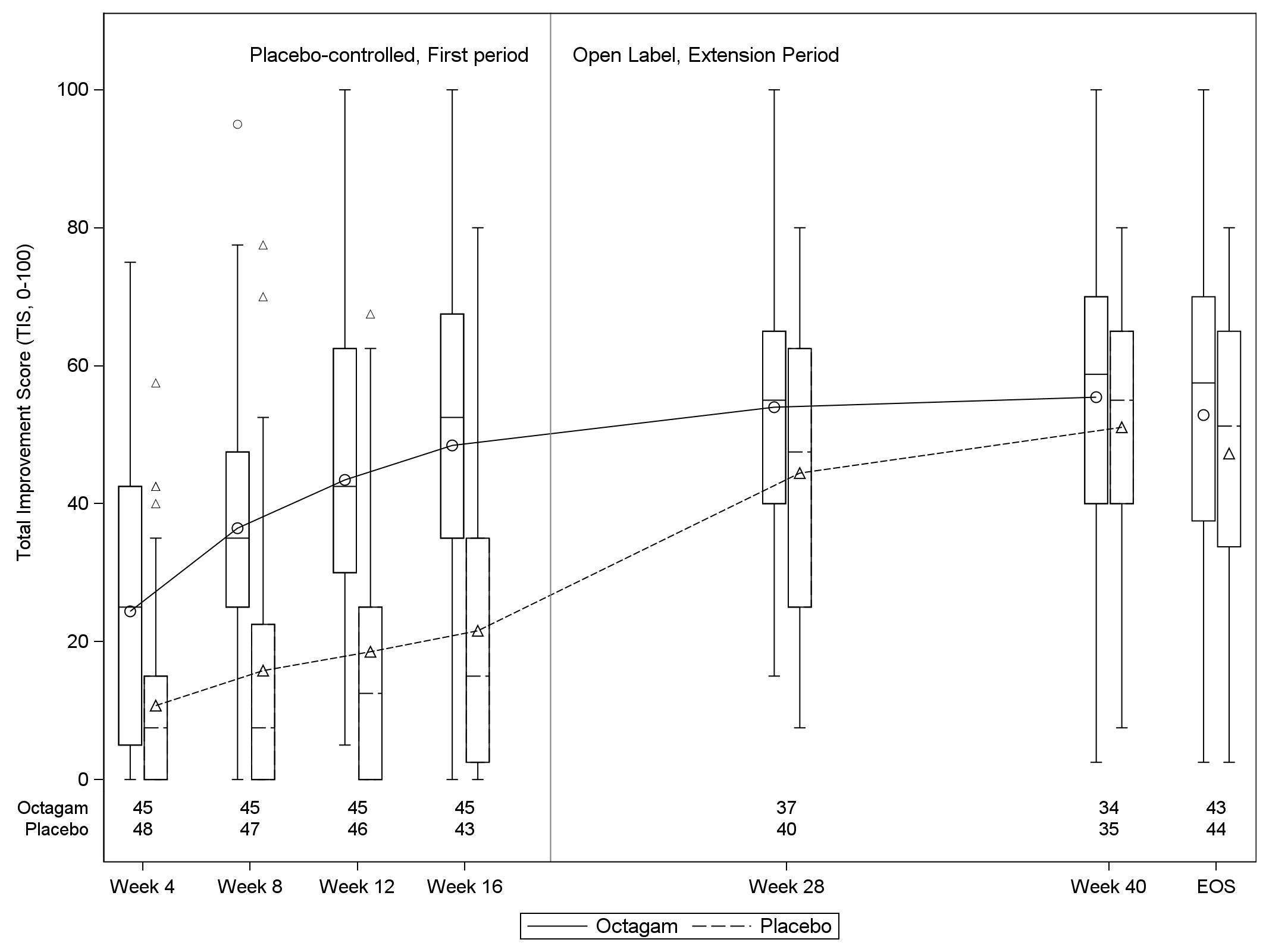 Fig 2: Mean Total Improvement Score (TIS) from week 4 to 40, among the two treatment arms
Fig 2: Mean Total Improvement Score (TIS) from week 4 to 40, among the two treatment arms
To cite this abstract in AMA style:
Aggarwal R, Charles-Schoeman C, Schessl J, Bata-Csorgo Z, Dimachkie M, Griger Z, Moiseev S, Oddis C, Schiopu E, Vencovský J, Irene B, Elisabeth C, Levine T, ProDERM Investigators a. Efficacy and Safety of IVIg (Octagam 10%) in Patients with Active Dermatomyositis. Results of a Randomized, Double-Blind, Placebo-Controlled Phase III Trial (ProDERM Study) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-ivig-octagam-10-in-patients-with-active-dermatomyositis-results-of-a-randomized-double-blind-placebo-controlled-phase-iii-trial-proderm-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-ivig-octagam-10-in-patients-with-active-dermatomyositis-results-of-a-randomized-double-blind-placebo-controlled-phase-iii-trial-proderm-study/
