Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The pathogenesis of rheumatoid arthritis (RA) is unclear, and additional therapeutic approaches are needed. The improvement of RA disease activity during pregnancy, and flares during the postpartum has been described. Control of the reproductive axis originates in the hypothalamus with the periodic pulsatile release of gonadotropin-releasing hormone (GnRH). Non-reproductive functions of GnRH include stimulation of immune responses (1), and this hormone has shown proinflammatory effects. A proof-of-concept, double blind, randomized controlled trial of GnRH antagonism in RA showed promising results, including a significant reduction in TNF (2). Here, the efficacy and safety of long-term GnRH antagonism therapy to patients with severe, biologic refractory RA is reported.
Methods: Nine patients (8 females and 1 male, mean age 59 years, mean disease duration 22 years, baseline mean DAS28CRP 6.79) with biologic refractory RA, fulfilling the ACR 2010 criteria, received off-label subcutaneous GnRH antagonist treatment in the form of either cetrorelix 0.75mg daily or degarelix 240mg loading dose, tapered down to 80mg approximately every 2 weeks. 12 week efficacy and safety data are reported. All patients were primary non-responders to the last biologic therapy administered. Stable concomitant csDMARDs and oral steroids were allowed. No intra-articular injections were received.
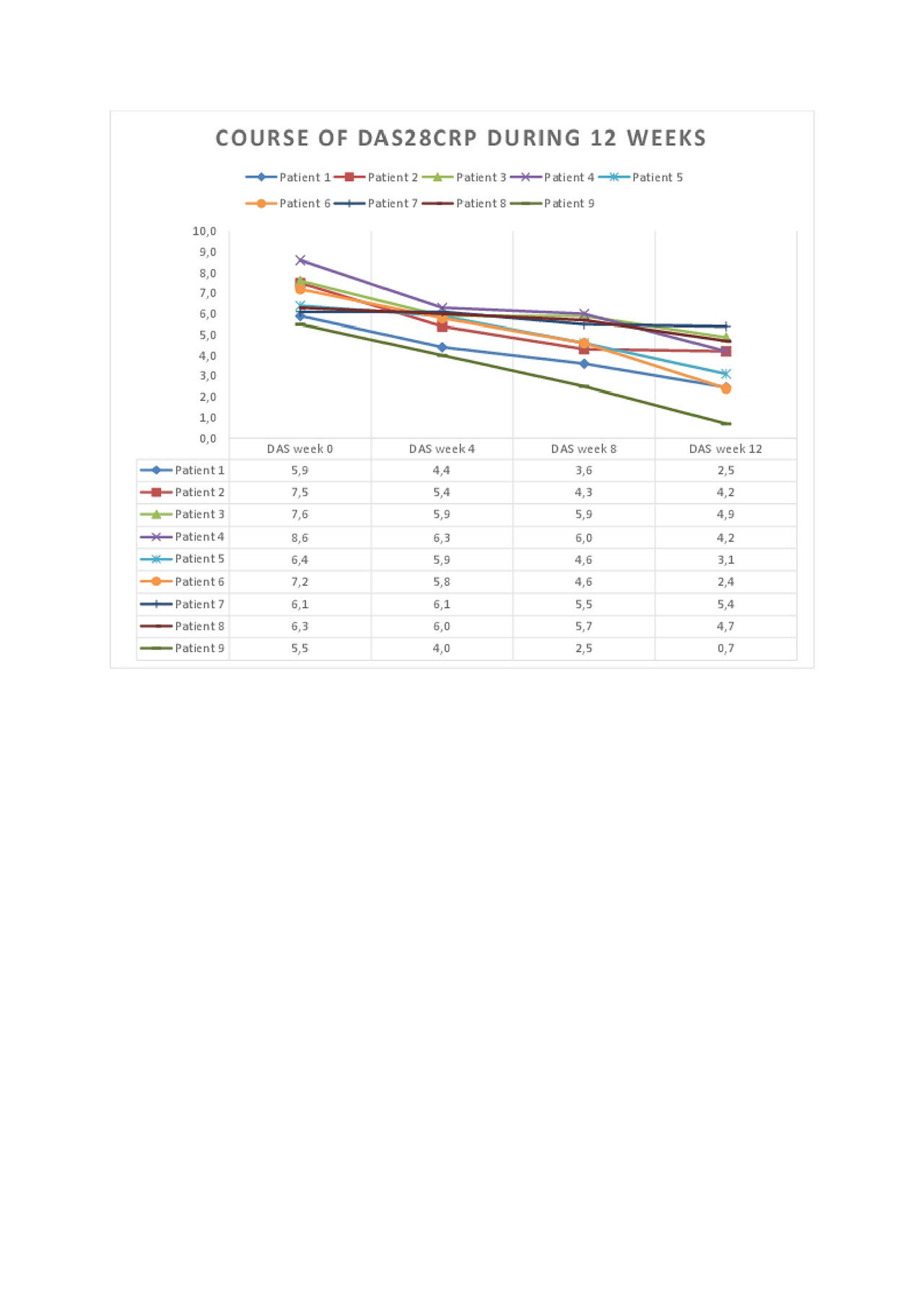
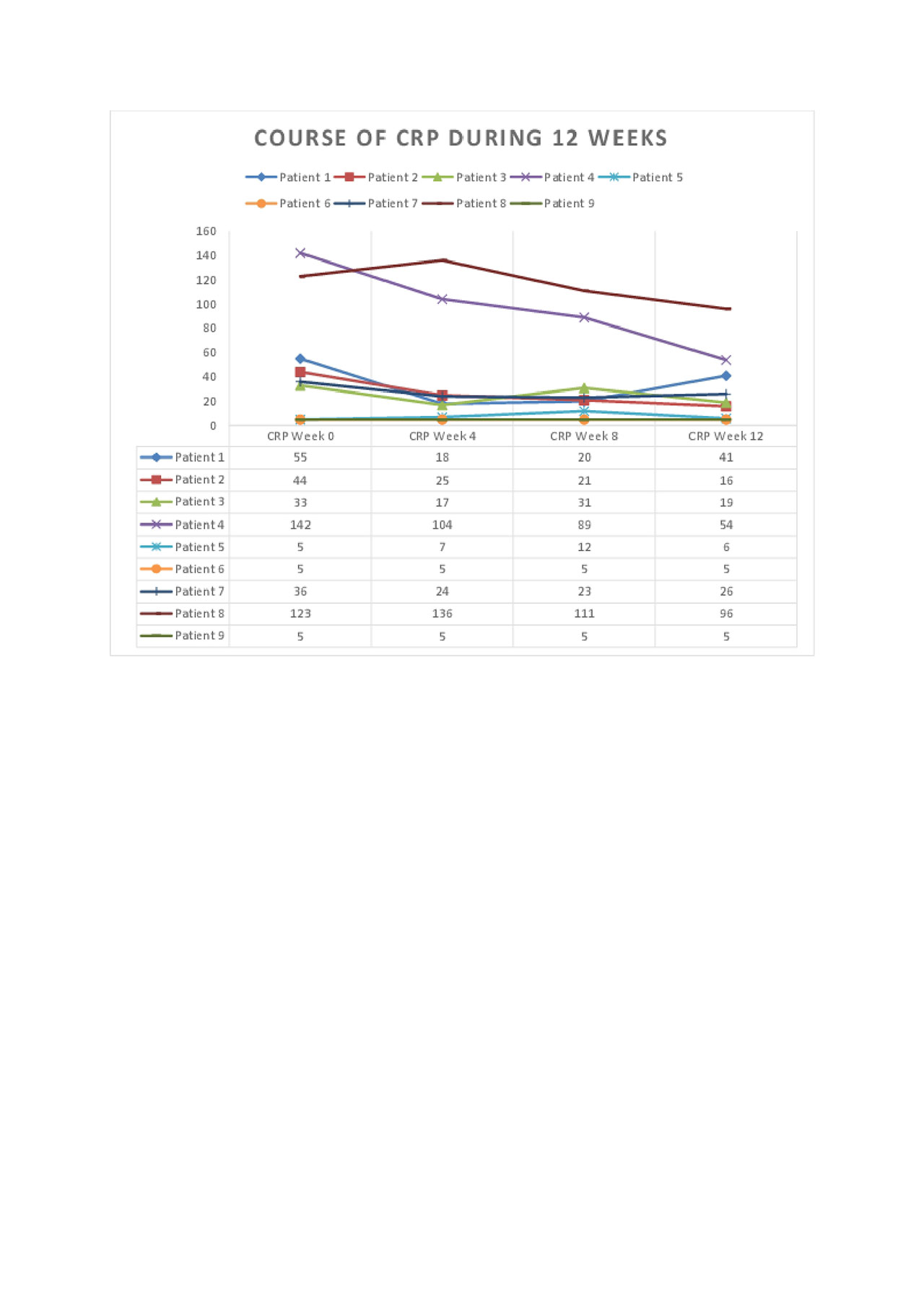
Results: DAS28CRP was significantly reduced (p< 0.001, Figure 1) at week 12 compared to baseline. The clinical and laboratory courses during 12 weeks are shown. All outcome measures were reduced, including joint counts, morning stiffness, MHAQ, and VAS patient reported outcomes. GnRH antagonism was well tolerated during this period, although hot flushes and mild to moderate injection site reactions were observed. Patient 3 developed a skin abscess on her foot where she had previously had several infections prior to GnRH antagonist therapy.
Conclusion: This data indicates that long-term GnRH antagonism may be beneficial in therapy-resistant RA patients. A plausible explanation involves the interruption of GnRH signaling on peripheral T cells. Further randomized controlled trials are required to verify these results and further assess safety.
To cite this abstract in AMA style:
Kass A, Gulseth H, Zettel C. Efficacy and Safety of Gonadotropin-Releasing Hormone Antagonism in Severe Biologic Refractory Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-gonadotropin-releasing-hormone-antagonism-in-severe-biologic-refractory-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-gonadotropin-releasing-hormone-antagonism-in-severe-biologic-refractory-rheumatoid-arthritis/