Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune‐mediated rheumatic diseases (IMRDs) are chronic inflammatory disorders driven by multiple overlapping immunological pathways. Although biologic DMARD monotherapy has substantially improved outcomes, a subset of patients remains refractory due to compensatory “immune escape” mechanisms and incomplete pathway inhibition. Off‐label combination therapy targeting two distinct immune axes has been proposed to overcome these challenges, yet real‐world data on its efficacy and safety are sparse and not reflected in current guidelines.
Methods: We conducted a retrospective, cross-sectional study at a tertiary reference center (CEIMI, Hospital General Universitario Gregorio Marañón, Madrid) including twenty adult patients (13 women; mean age 48.5 years) diagnosed with rheumatoid arthritis (n = 5), psoriatic arthritis (n = 5), psoriasis (n = 1), spondyloarthritis (n = 3), inflammatory bowel disease (n = 2), systemic lupus erythematosus (n = 2), or juvenile idiopathic arthritis (n = 2). All had failed a median of four prior st/bDMARDs (6.6 in RA) and initiated off-label dual therapy with agents targeting distinct immune pathways. Clinical, laboratory, efficacy (remission or low disease activity [LDA] by standardized indices), and safety data were extracted at baseline and at 1, 3, 6, 12, and up to 36 months post-CBT. Descriptive statistics summarized outcomes.
Results: At CBT initiation, 65 % of patients had high disease activity and were on glucocorticoids. Sixteen regimens were used; the most common paired a CD20 inhibitor with a JAK inhibitor (25 %), and rituximab featured in nine. Mean exposure was 27 months (range 2–68), and 80 % remained on CBT. By month 1, 65 % achieved remission or low disease activity (LDA), rising to 87 % at month 3. At 6 months (n=12), 100 % were in remission/LDA and 78 % had reduced or stopped glucocorticoids. Sustained rates were 71 % at 12 and 91 % at 18 months.All five RA patients reached remission/LDA by month 3, with one flare at 24 months. Both RF-positive JIA cases improved by month 1; one achieved LDA at month 3 and both discontinued steroids. PsA patients showed durable PASI and DAPSA score improvements. Two of three SpA patients remained in remission; one experienced worsened gastrointestinal symptoms. Both IBD patients improved clinically; one ceased CBT upon stable remission. In SLE patients, SLEDAI-2K scores dropped by 9–10 points at month 1. Four regimens (20 %) were discontinued—one for progressive neutropenia (resolved after stopping rituximab + belimumab), one for surgery, one at patient request, and one due to sustained remission.
Conclusion: In this real-world cohort of refractory IMRD patients, dual biologic/synthetic targeted therapy produced rapid, sustained remission or LDA across diverse diagnoses, enabled glucocorticoid tapering, and demonstrated a manageable safety profile. These findings warrant prospective studies to define the role of multidimensional pathway blockade in difficult-to-treat rheumatic disease.
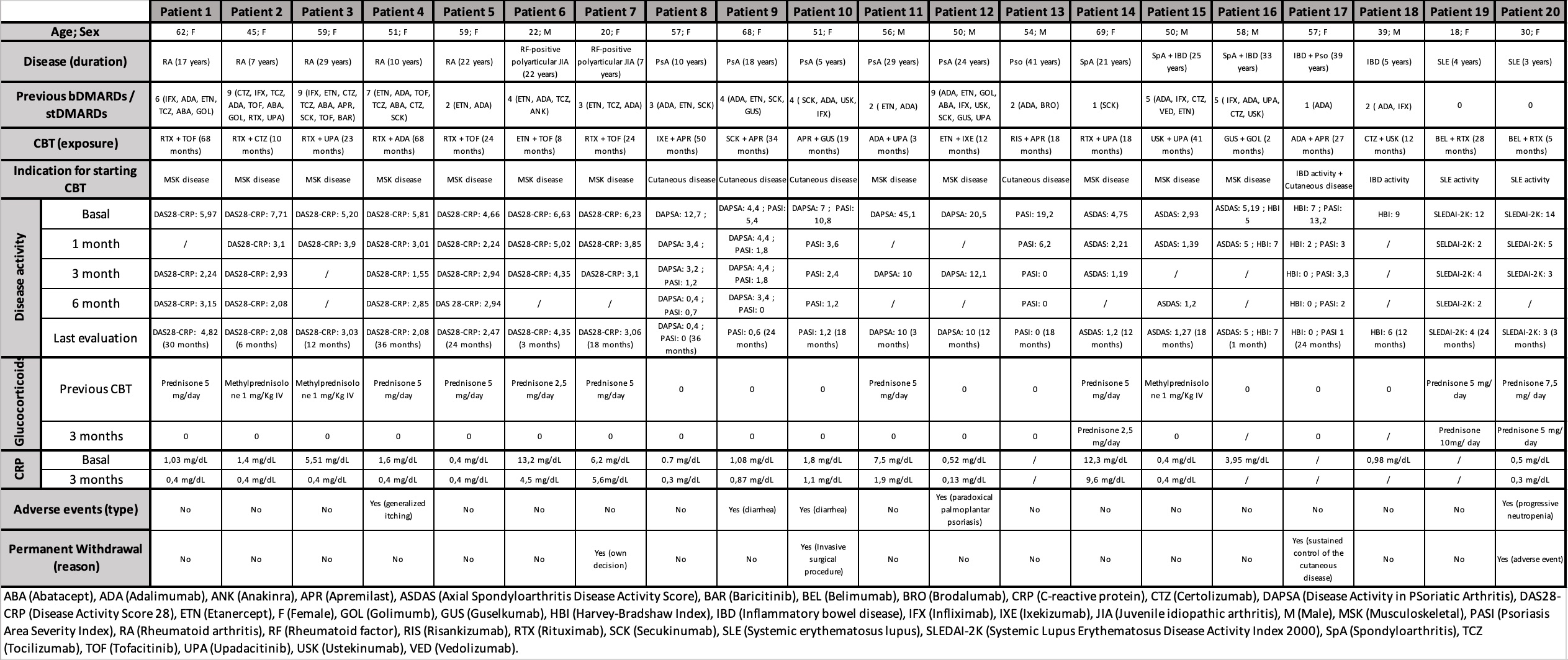 Main clinical and serological features and outcomes of patients under CBT
Main clinical and serological features and outcomes of patients under CBT
To cite this abstract in AMA style:
Bizzarri F, Menchén Viso L, Chamorro De Vega E, Baniandrés Rodríguez O, Nieto J. Efficacy and safety of dual biological therapy in the treatment of rheumatic diseases: experience from a single highly specialized center. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-dual-biological-therapy-in-the-treatment-of-rheumatic-diseases-experience-from-a-single-highly-specialized-center/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-dual-biological-therapy-in-the-treatment-of-rheumatic-diseases-experience-from-a-single-highly-specialized-center/
