Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Avacopan, an oral C5a receptor inhibitor, was demonstrated to be an alternative to glucocorticoids (GC) in the treatment of microscopic polyangiitis (MPA)/granulomatosis with polyangiitis (GPA) in the ADVOCATE trial and was approved in several countries including Japan. However, there are still few reports as for the efficacy and safety of avacopan in real-world settings. Additionally, the effect of avacopan on serum biomarkers is also unclear. Therefore, we aimed to clarify the efficacy and safety of avacopan over 12 months in Japanese patients with MPA/GPA in a real-world setting, as well as changes in serum antineutrophil cytoplasmic antibody (ANCA) levels.
Methods: Patients with MPA/GPA who started remission induction therapy with GC plus rituximab or cyclophosphamide at our institution from January 2017 to June 2023 were included in this retrospective study. To evaluate the efficacy and safety of avacopan, patients were divided into two groups: one group received avacopan in combination with remission induction therapy (avacopan group) and the other group did not (non-avacopan group). The achievement rate of BVAS=0, daily GC dose (prednisolone-equivalent), and the incidence of adverse events (AEs) until 12 months after treatment were investigated and compared between the two groups using the Wilcoxon rank-sum test and Fisher’s exact test. The differences in changes in serum ANCA levels between the two groups were also analyzed using the Wilcoxon rank-sum test.
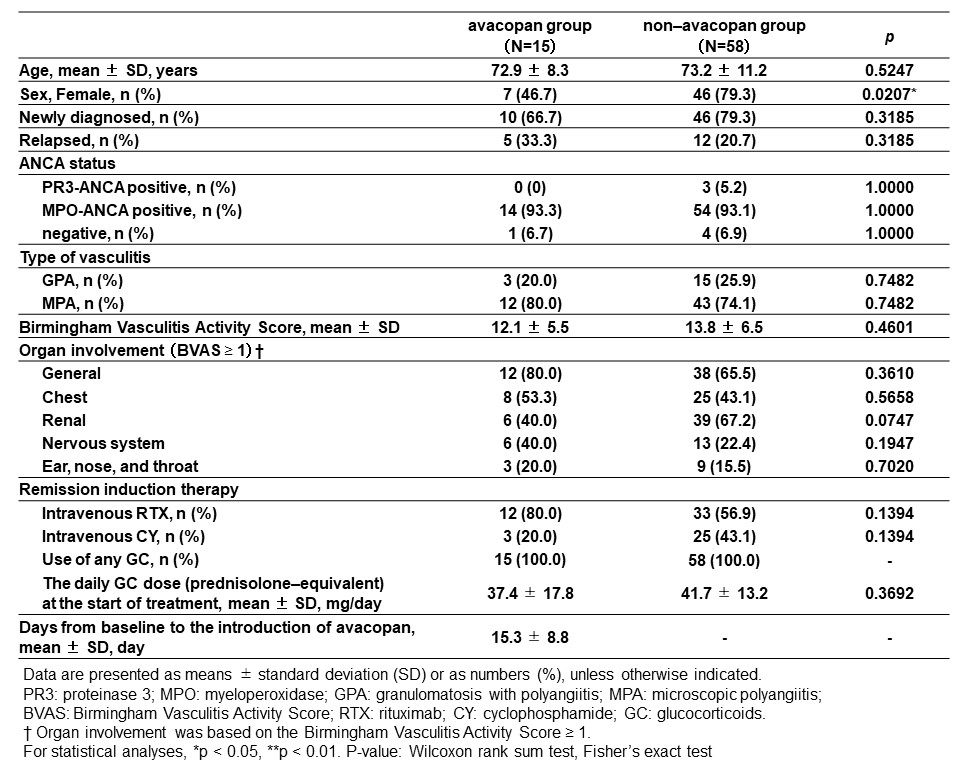
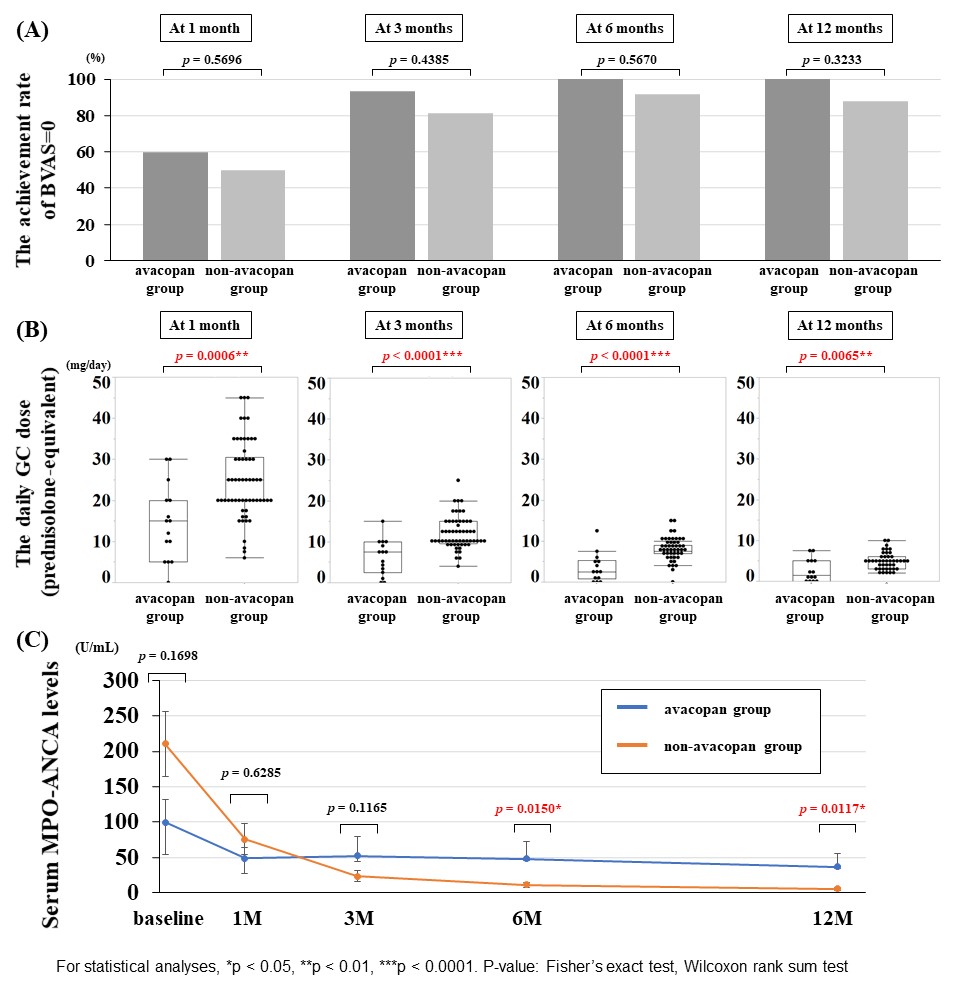
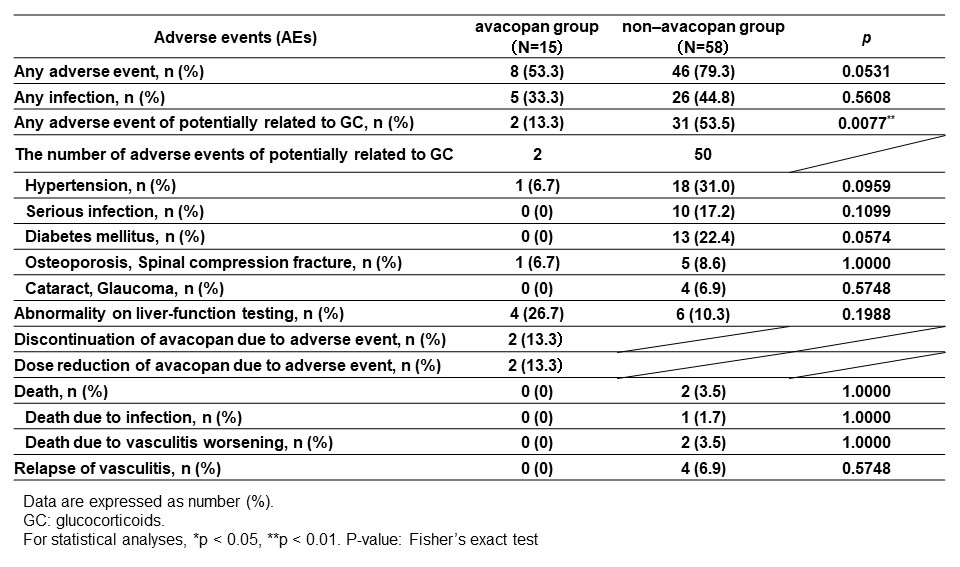
Results: A total of 73 patients with MPA/GPA were included, 15 in the avacopan group and 58 in the non-avacopan group. There were no differences between the two groups in age, ANCA status, proportion of MPA/GPA, or type of remission induction therapy, except for gender proportion (Figure 1). The achievement rate of BVAS=0 at 1, 3, 6 and 12 months was not significantly different between the avacopan and non-avacopan groups (60.0% vs. 50.0%, 93.3% vs. 81.0%, 100% vs. 91.8%, 100% vs. 87.8%, respectively) (Figure 2(A)). On the other hand, the mean daily GC dose at 1, 3, 6 and 12 months was significantly lower in the avacopan group than in the non-avacopan group (14.5 ± 9.2 mg/day vs. 24.8 ± 9.4 mg/day, 6.1 ± 4.4 mg/day vs. 12.0 ± 4.0 mg/day, 3.5 ± 3.5 mg/day vs. 7.8 ± 2.8 mg/day, 2.7 ± 2.8 mg/day vs. 4.9 ± 2.0 mg/day, respectively) (Figure 2(B)). Serum MPO-ANCA decreased in both groups after treatment, however MPO-ANCA levels at 6 and 12 months were significantly lower in the non-avacopan group (Figure 2(C)). The incidence of GC-related AEs (defined as hypertension, diabetes, osteoporosis, cataract and glaucoma, and severe infection) within 12 months was significantly lower in the avacopan group than in the non-avacopan group (13.3% vs. 53.5%, p< 0.01). Four patients in the avacopan group had drug-induced liver injury, leading to dose reduction of avacopan in 2 and discontinuation of avacopan in 2 (Figure 3).
Conclusion: This study demonstrated that avacopan is an effective medication for reducing the GC dose and the GC-related AEs in treatment for MPA/GPA over 12 months in a real-world setting. On the other hand, avacopan-induced liver injury should be noted. The results also suggested that avacopan have a small effect on the decrease in serum MPO-ANCA levels.
To cite this abstract in AMA style:
Ushio Y, Shimada H, miyagi t, Sugihara K, Mizusaki M, Mino R, yamaguchi H, Manabe N, Nakashima S, Dobashi H. Efficacy and Safety of Avacopan over 12 Months in Japanese Patients with MPA/GPA: A Single-center Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-avacopan-over-12-months-in-japanese-patients-with-mpa-gpa-a-single-center-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-avacopan-over-12-months-in-japanese-patients-with-mpa-gpa-a-single-center-study/