Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The randomized, double-blind, double-dummy, controlled Phase 3 ADVOCATE trial tested whether avacopan, an oral selective C5a receptor inhibitor approved for the treatment of ANCA-associated vasculitis (AAV), could replace a glucocorticoid (GC)-tapering regimen. Randomization was stratified according to vasculitis disease status (newly diagnosed or relapsing), ANCA status (anti-proteinase 3 (PR3) positive or anti-myeloperoxidase positive), and immunosuppressive treatment (cyclophosphamide (CYC) or rituximab (RTX)). American College of Rheumatology/Vasculitis Foundation guidelines recommend induction treatment with RTX over CYC (Chung SA et al, Arthritis Care Res (Hoboken) 2021). For relapsing disease, EULAR guidelines recommend RTX (Hellmich B et al,Ann Rheum Dis 2023). The objective of this study was to evaluate the efficacy and safety of avacopan in patients in the RTX stratum of the ADVOCATE trial.
Methods: Primary efficacy endpoints were the proportion of patients achieving disease remission at week 26 and sustained remission at week 52 (Birmingham Vasculitis Activity Score (BVAS) of 0 and no GC use for AAV 4 weeks before measurement). RTX was administered intravenously 375 mg/m2 once weekly for 4 weeks beginning on Day 1. The protocol did not include repeat dosing of RTX, according to its approved labeled regimen at the time.
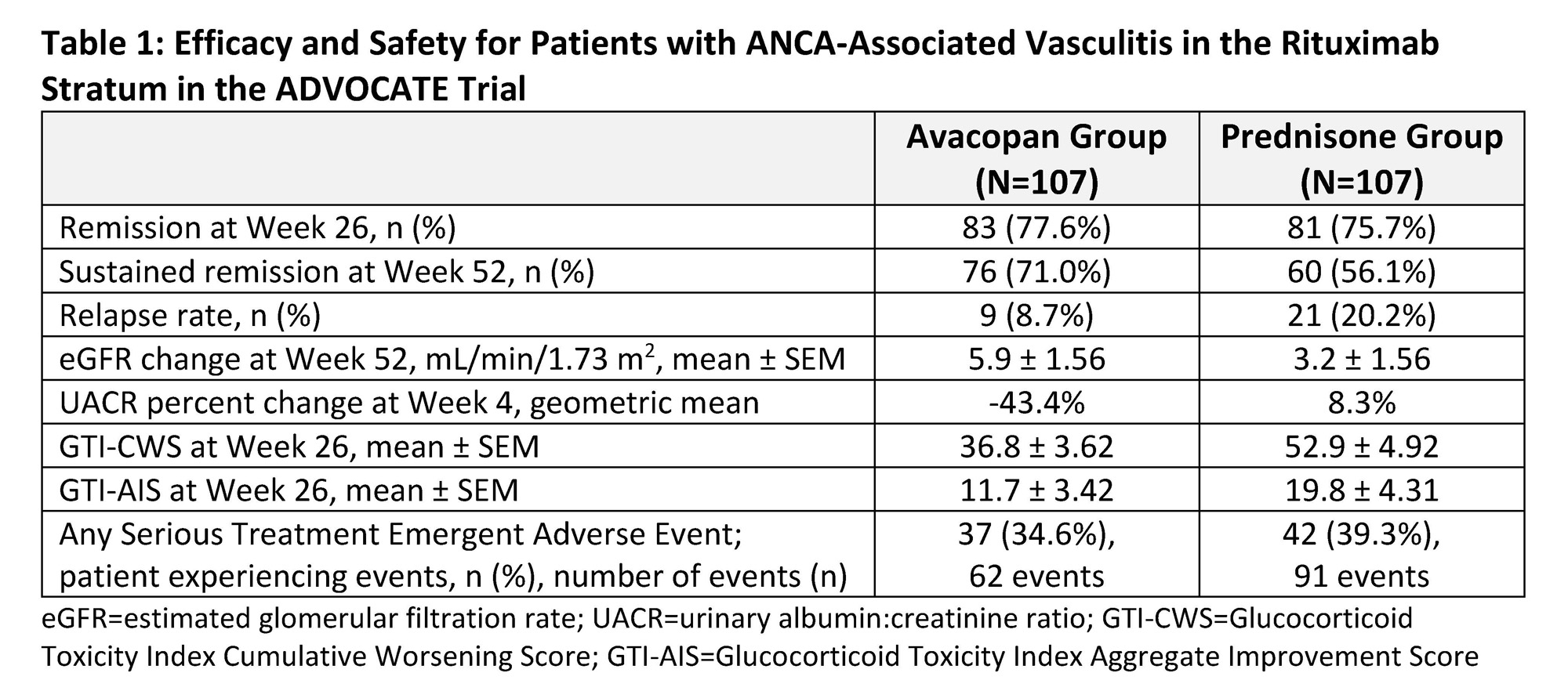
Results: A total 214 of 330 patients (64.8%) comprised the RTX stratum. At baseline, patients were 60 years old (mean), ~76% had renal vasculitis (based on BVAS), ~46% were PR3-ANCA positive, and ~58% were newly diagnosed. Men constituted 57.0% of the avacopan group and 48.6% of the prednisone group. Baseline estimated glomerular filtration rate (eGFR) was 57.1±32.2 and 56.0±33.4 mL/min/1.73 m2 (mean±SD), for the avacopan and prednisone groups, respectively. Remission at week 26 was 77.6% in the avacopan group and 75.7% in the prednisone group (Table 1). Sustained remission at week 52 was 71.0% in the avacopan group and 56.1% in the prednisone group (Table 1), estimated common difference, 16.5 percentage points; 95% confidence interval, 4.3 to 28.6. Additionally, there were numerical improvements in relapse rate, albuminuria, and lower GC-associated toxicity in the avacopan group versus the prednisone group. The proportion of patients experiencing a serious treatment emergent adverse event (TEAE) was 34.6% (n = 37) with 62 events in the avacopan group compared to 39.3% (n = 42) with 91 events in the prednisone group (Table 1).
Conclusion: This analysis from the ADVOCATE trial of patients with AAV receiving RTX showed a significantly increased sustained remission rate at week 52 in the avacopan group compared to the prednisone group. Compared to the prednisone group, patients in the avacopan group had numerical improvements in remission at week 26, relapse rate, eGFR, albuminuria, and less GC toxicity. There was no increase in serious TEAEs in patients treated with avacopan. These results demonstrate the efficacy of avacopan for achieving and sustaining remission in patients with AAV treated with RTX.
To cite this abstract in AMA style:
Geetha D, Dua A, Yue H, Salvarani C, Jayne D, Merkel P. Efficacy and Safety of Avacopan in Patients with ANCA-Associated Vasculitis Receiving Rituximab in a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-avacopan-in-patients-with-anca-associated-vasculitis-receiving-rituximab-in-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-avacopan-in-patients-with-anca-associated-vasculitis-receiving-rituximab-in-a-phase-3-trial/