Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In the randomized,
double-blind, placebo-controlled, Phase III CRYSTAL trial, more patients taking
lesinurad 200 mg (LESU200) or 400 mg (LESU400), in combination with febuxostat
(FBX) 80 mg, achieved serum uric acid (sUA) <5.0mg/dL at 6 months versus FBX
+ placebo (PBO). Patients completing 12 months on study could enroll in an
extension study (NCT01808144). The
objective was to examine efficacy and safety in patients taking lesinurad combination
therapy for up to 2 years.
Methods: Patients on PBO+FBX in the core study were randomized to
LESU200+FBX (200CROSS) or LESU400+FBX (400CROSS). Patients randomized to LESU200+FBX
and LESU400+FBX in the core study were continued on their therapy (200CONT, 400CONT).
Primary endpoint was the proportion of patients experiencing complete
resolution (CR) of ≥1 target tophus (measurable tophus on hands/wrists
and/or feet/ankles 5–20mm in longest diameter, using Vernier calipers) during
core and extension studies by extension Month 12. The sum of areas for all
target tophi, proportion of patients achieving sUA <5.0mg/dL, and proportion
of patients experiencing gout flares requiring treatment (GFRT) were secondary
endpoints.
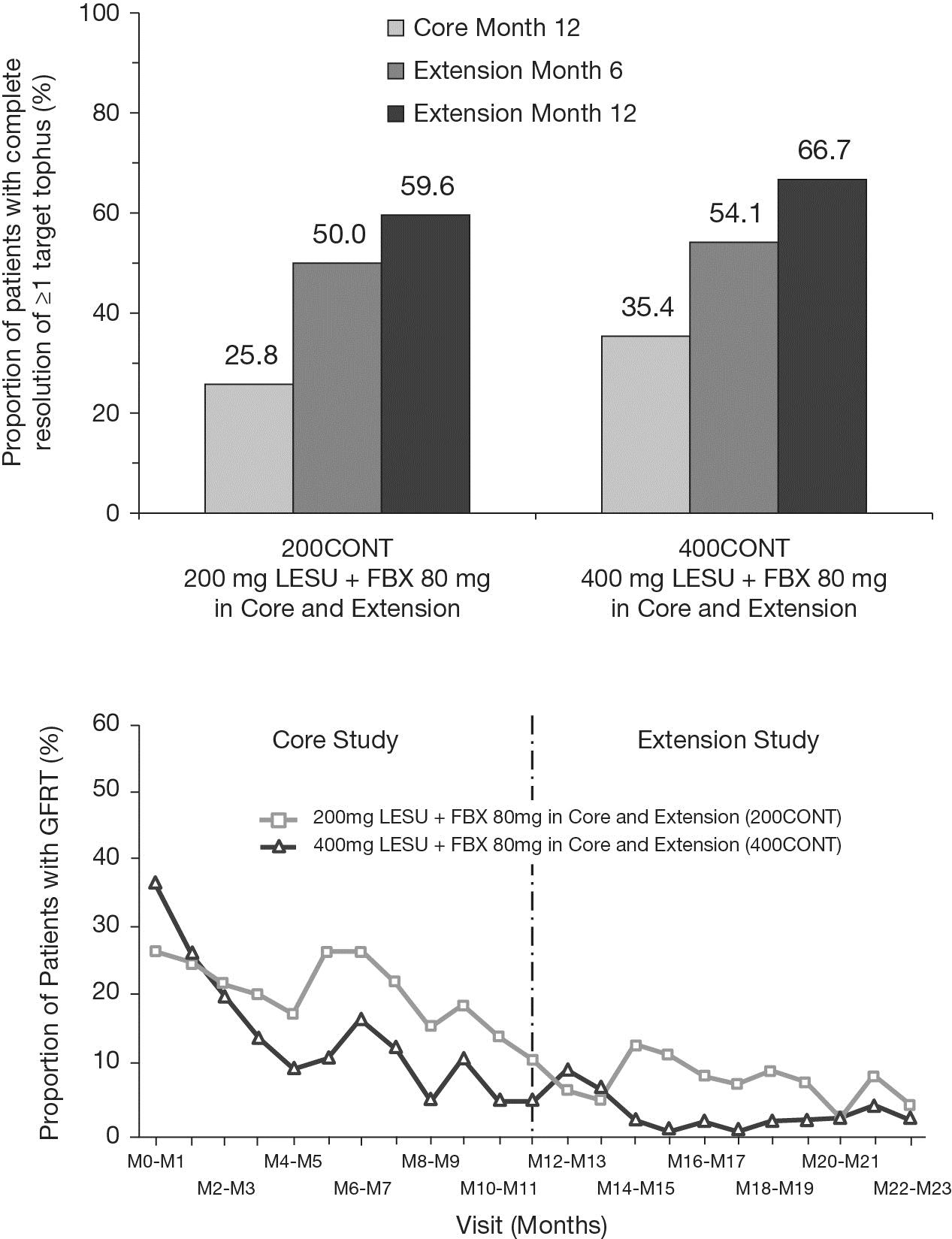
Results: Of 235 patients completing the core study, 196 enrolled in the extension:
200CONT (n=64), 200CROSS (n=33), 400CONT (n=65), and 400CROSS (n=34). For those
continuing core treatment (200CONT, 400CONT), 59.6% and 66.7% of patients had
CR of ≥1 target tophus (Figure), with 68.3% and 72.4% reductions in the sum of areas for
all target tophi, respectively, after 2 years. GFRT during extension occurred
in 32.8% and 13.8% of patients, with few patients having GFRT during the second
year (Figure). Target sUA levels <5.0mg/dL at Extension Month 12 were
achieved in 77.1% and 88.5% of patients. For crossover groups (200CROSS,
400CROSS respectively), 43.5% and 50.0% of patients had CR of ≥1 target tophus, with 64.1% and 44.1% reductions in target tophi area after 1
year of combination therapy. GFRT rates during extension were 37.5% and 38.2%,
with 79.2% and 71.4% of patients achieving target sUA <5.0 mg/dL. Treatment-emergent
adverse events (TEAEs) with extended exposure to lesinurad were generally
comparable between groups. The most common TEAEs for the CONT groups were serum
creatinine increase (10.9%) and bronchitis (7.0%); for the CROSS groups,
nasopharyngitis (13.4%) and headache (7.5%) were most common, with serum
creatinine increase occurring in 6.0% of patients.
Conclusion: Patients continuing on lesinurad
+ FBX therapy over 2 years continued to be at sUA target, with no attenuation
of sUA effect. Patients exhibited continued increase in complete resolution of
tophi, additional tophi area reduction, and reduction of GFRT over the second
year on study. AEs in the extension study were consistent with those observed
in the core study.
To cite this abstract in AMA style:
Dalbeth N, Jones G, Terkeltaub R, Khanna D, Kopicko J, Adler S, Bhakta N, Fung M, Storgard C, Baumgartner S, Perez-Ruiz F. Efficacy and Safety in Patients with Tophaceous Gout Receiving Lesinurad and Febuxostat Combination Therapy: Interim Analysis of an Extension Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-in-patients-with-tophaceous-gout-receiving-lesinurad-and-febuxostat-combination-therapy-interim-analysis-of-an-extension-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-in-patients-with-tophaceous-gout-receiving-lesinurad-and-febuxostat-combination-therapy-interim-analysis-of-an-extension-study/