Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Avacopan, a selective C5aR1 inhibitor, has demonstrated efficacy and safety over 52 weeks in patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. However, efficacy and safety data on avacopan beyond 52 weeks are limited. Here, we describe the experience with avacopan beyond 52 weeks from the EAP.
Methods: Safety data in patients with severe, active granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA) within the EAP were recorded in a global safety database from Feb 2019 – Apr 2023. Adverse events (AE) included a lack of effect and other events (i.e., relapse or worsening of disease).
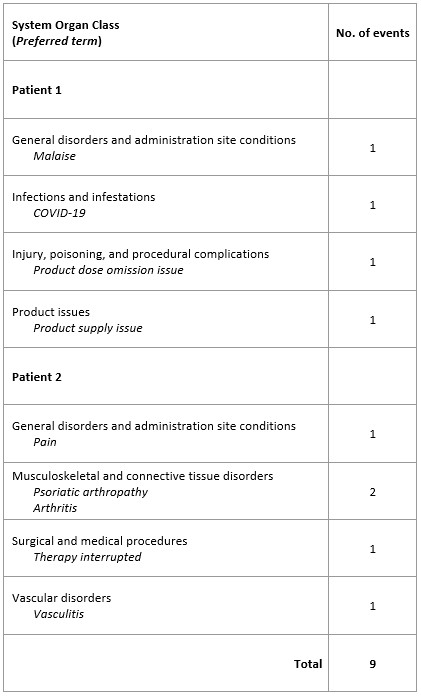
Results: A total of 19 patients were treated with avacopan beyond 52 weeks within the EAP. Average age was 47 years, with 13 patients (68%) diagnosed with GPA and 6 (32%) with MPA. The median duration of therapy was 17 months (range 12–45). A total of 9 AEs were recorded in 2 patients (10.6%) (Table 1). One vasculitis flare was recorded 6 months after avacopan initiation and coincided with an unintended dose reduction to 20 mg BID, due to a product supply issue during COVID. The event was well-managed with rituximab, with no additional use of glucocorticoids, and avacopan 30 mg BID was reinstated. No further cases of a lack of effect, worsening of disease, or disease relapse were reported. Data regarding concomitant medications did not indicate a decline in the patients’ status during treatment. No treatment discontinuations due to AEs were recorded.
Conclusion: These results suggest that continuation of avacopan beyond 52 weeks is generally well-tolerated in patients with GPA and MPA and may be effective in terms of disease control. Limitations of this program include low patient number, potential underreporting, and incomplete data.
To cite this abstract in AMA style:
Alberici F, Salvarani C, Chan C, Obergfell A, Popov T. Efficacy and Safety Experience with Avacopan Beyond 52 Weeks in the Early Access Program (EAP) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-experience-with-avacopan-beyond-52-weeks-in-the-early-access-program-eap/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-experience-with-avacopan-beyond-52-weeks-in-the-early-access-program-eap/