Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: People with knee OA (KOA) desire therapies which delay or reverse disease progression supporting the need for disease-modifying OA drugs (DMOADs). Sprifermin, truncated recombinant human fibroblast growth factor‑18, demonstrated dose-dependent increased cartilage thickness in KOA in the Phase 2 FORWARD study. Improvement from baseline (BL) in WOMAC Pain and Function occurred in all sprifermin study arms with a lack of differentiation from placebo (PBO), potentially due to including participants (pts) not at risk of KOA progression. Minimum clinically meaningful changes (improvement or worsening) in WOMAC Pain and Function have been defined as ∼10 points (0-100 scale). A post hoc analysis of FORWARD identified a subgroup at risk (SAR) enriched for disease progression, in which improvement in WOMAC Pain ≥10 points for sprifermin over PBO was seen by Year (Y) 3. Worsening WOMAC Pain ≥10 points has been predictive of worsening function, structural progression, and knee replacement in KOA natural history studies. This post hoc analysis of the FORWARD study evaluates the effect of sprifermin on the symptomatic progression of KOA.
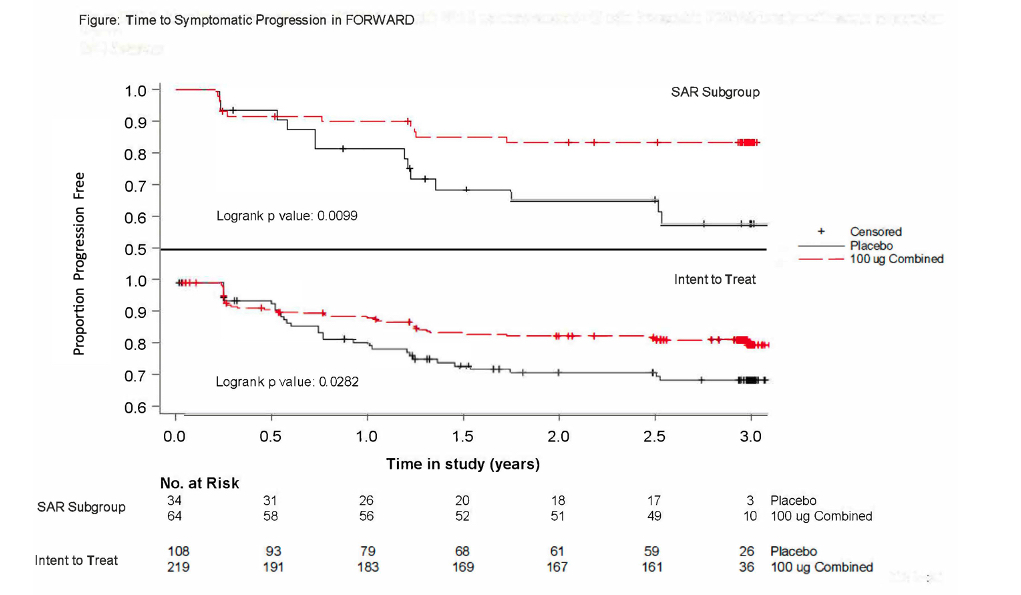
Methods: Pts were randomized 1:1:1:1:1 to intra-articular PBO or sprifermin 30 µg or 100 µg every 6 or 12 months (Q6M or Q12M) for 18 months and followed through Y5. WOMAC was collected Q3M and MRI Q6M through Y2 and then Q6M and Q12M, respectively. In this Kaplan-Meier analysis, time to symptomatic progression (ie, first occurrence of worsening [increase] of WOMAC Pain of ≥10 points with no improvement [≤9 point decrease] in WOMAC Function) was evaluated through Y3. All treatment arms of the intent-to-treat population (ITT) and SAR (ie, BL WOMAC Pain 40-90, minimum joint space width 1.5-3.5 mm) were analyzed, as well as the sprifermin 100 µg groups combined for additional power. Time to symptomatic progression was also analyzed by changes in cartilage thickness (decrease or no change/increase) for those with evaluable MRIs at BL and ≥1 post-BL timepoint, ie, modified (m) ITT and mSAR.
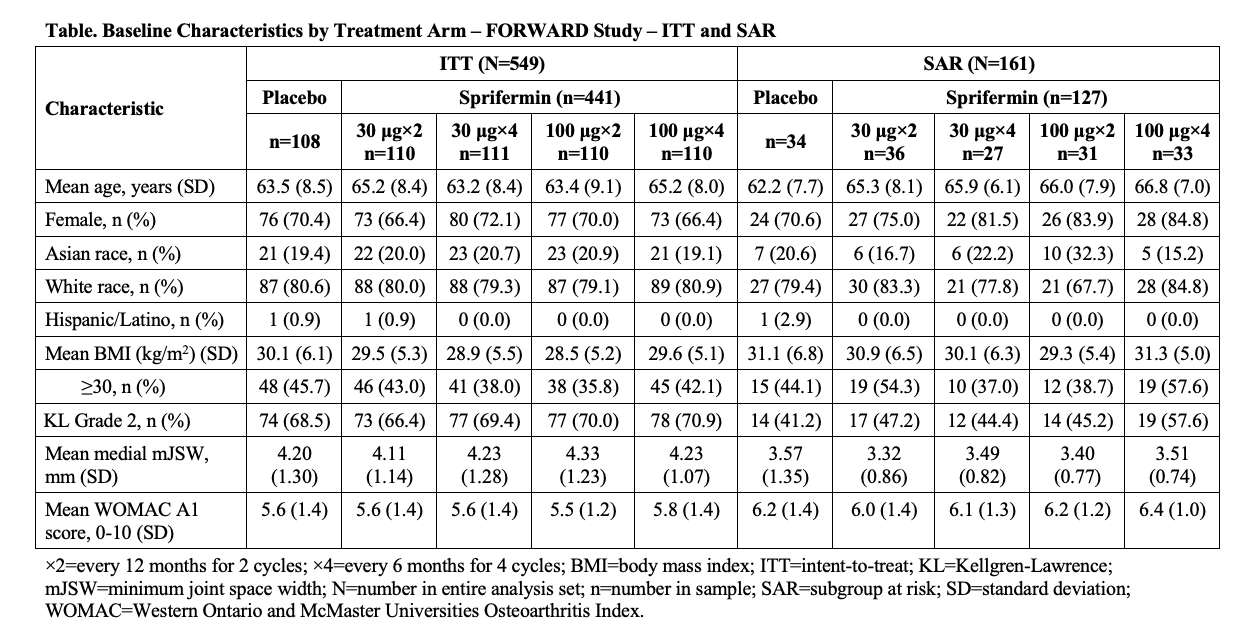
Results: Baseline characteristics were comparable across the FORWARD ITT and SAR treatment arms (Table). Sprifermin showed dose-dependent benefits in the time to symptomatic progression compared to PBO, with clinically meaningful and statistically significant separation from PBO for the sprifermin 100 µg groups combined (nominal logrank p‑value ITT < 0.05, SAR < 0.01; HR [95% CI] ITT 0.59 [0.37, 0.95], SAR 0.35 [0.16, 0.81]) (Figure); separation from PBO was more pronounced in the SAR compared to the ITT. The sprifermin 100 µg combined group also demonstrated less symptomatic progression than PBO regardless of an increase or no change/decrease in cartilage thickness in the mSAR and mITT.
Conclusion: These post hoc results support that sprifermin may prevent symptomatic progression of KOA, with benefits seen in both ITT and SAR populations for the sprifermin 100 µg combined group. Further study is needed to confirm the symptomatic and structural benefits of sprifermin. As differences between the sprifermin- and PBO-treated groups were detectable in a 3‑year time frame, time to symptomatic progression of KOA could be a meaningful endpoint for a DMOAD trial.
To cite this abstract in AMA style:
Conaghan P, Katz N, Hunter d, Hochberg M, Guermazi A, Somberg K, Clive J, Johnson M, Goel N. Effects of Sprifermin on a Novel Outcome of Osteoarthritis Symptom Progression: Post Hoc Analysis of the FORWARD Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effects-of-sprifermin-on-a-novel-outcome-of-osteoarthritis-symptom-progression-post-hoc-analysis-of-the-forward-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-sprifermin-on-a-novel-outcome-of-osteoarthritis-symptom-progression-post-hoc-analysis-of-the-forward-study/