Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease caused by genetic and environmental factors characterised by joint inflammation. The JAK-STAT inhibitors (jakinibs) are among the therapeutic options.
Methods: Seventy-five patients treated with jakinibs were recruited, 20 healthy donors and, 20 RA patients treated with biological DMARDs, both paired by sex and age with the jakinibs group, were enrolled as controls. Peripheral blood mononuclear cells were isolated and analysed by multiparametric flow cytometry to characterise the immunophenotype of different subsets of the immune system.
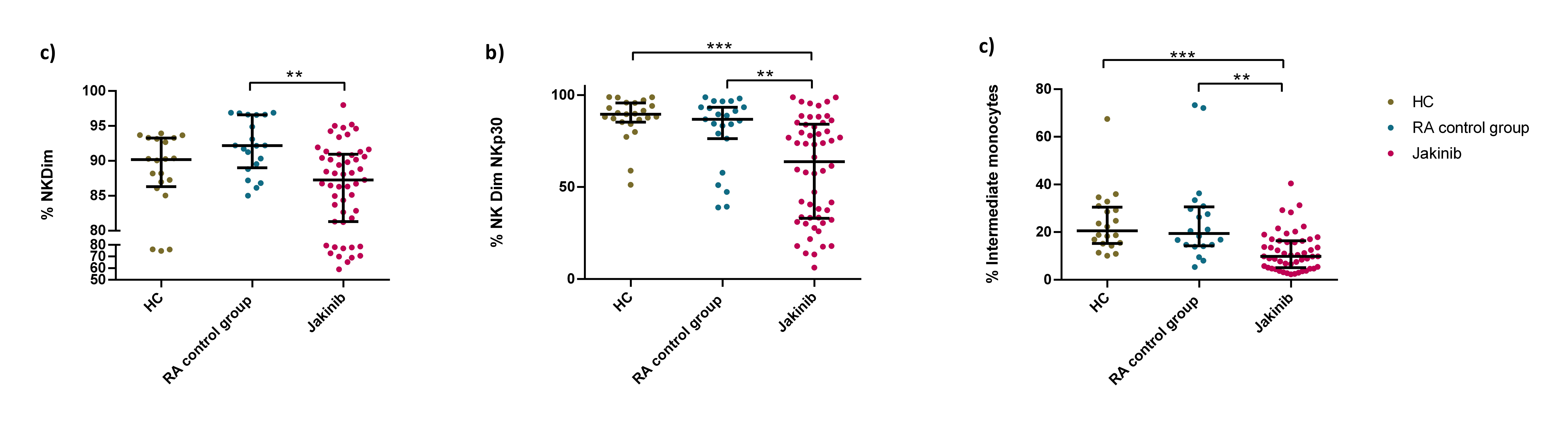
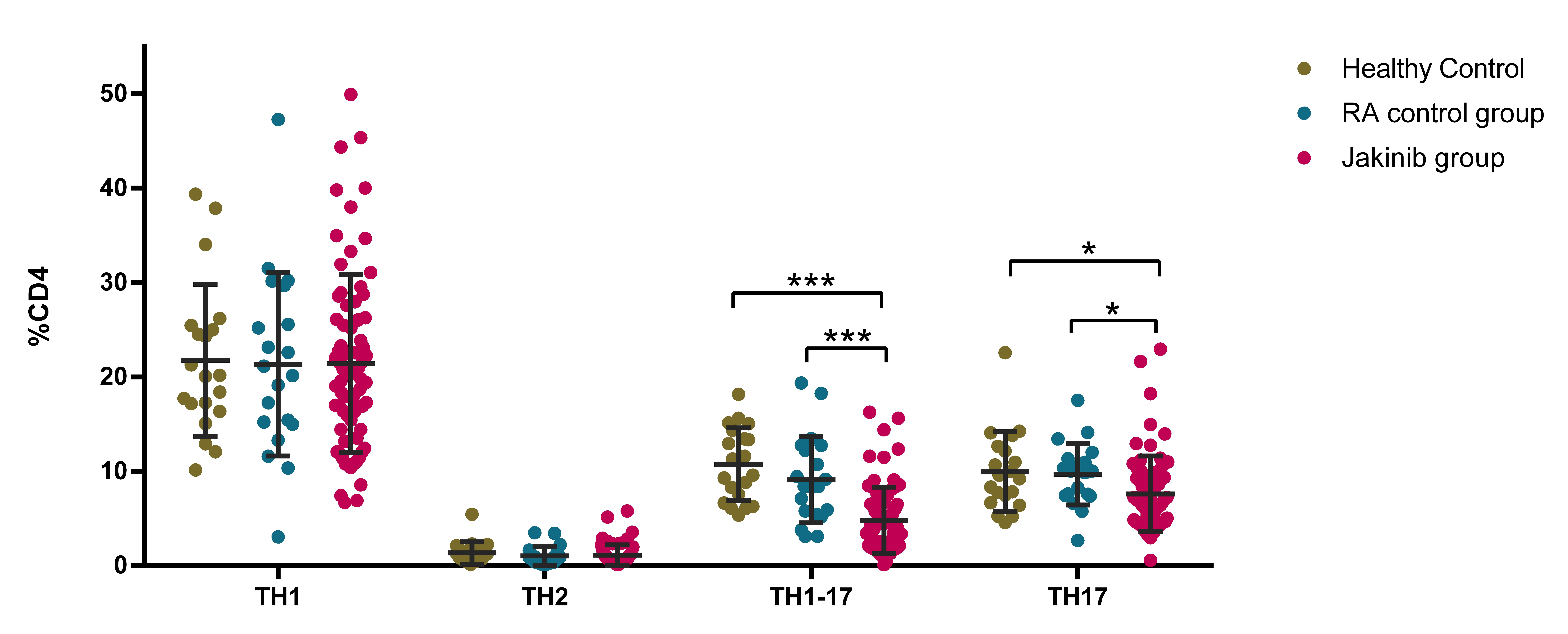
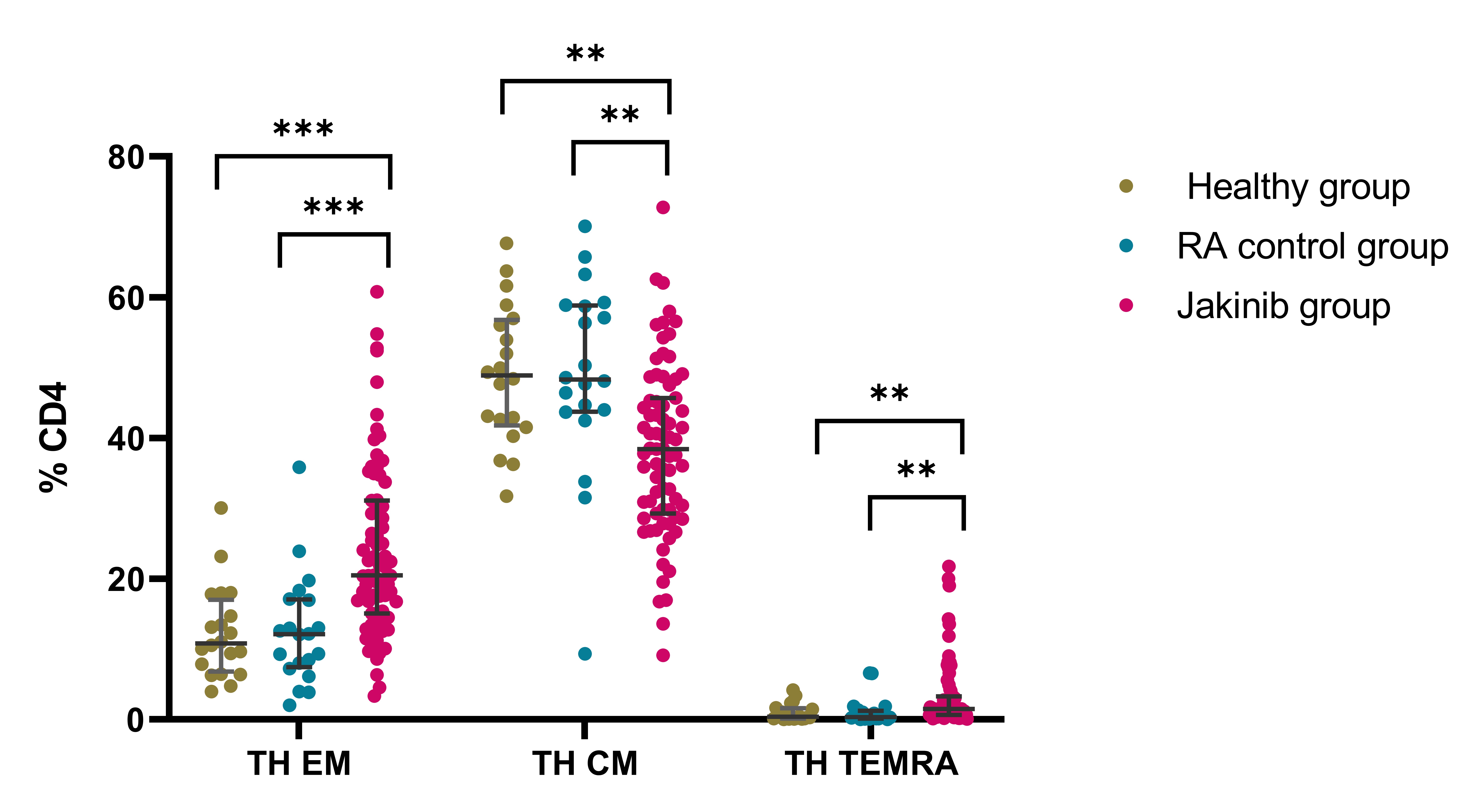
Results: Within the jakinib group, 36 (48 %) patients were treated with baricitinib (Bari), 13 (17.3%) tofacitinib (Tofa), 19 (24 %) filgotinib (Filgo) and 8 (10.7%) with upadacitinib (Upa); in the RA control group 11 patients were treated with tocilizumab and 9 with abatacept. A significant decrease in the percentage of cytotoxic NK Dim (CD56+CD16+) subset was observed in the jakinib group in comparison to the RA group [87.3 (81.3-90.9) vs 92.2 (89.2-96.6), p=0.001]. There were significant differences between jakinib group and both healthy and RA patients in the percentage of activated NK Dim expressing Nkp30 [59.51 (32.2-80.3) vs 89.7 (84.9-95.8) and 85.3 (67.1-93.2), respectively; p values: < 0.001 and 0.007]. In addition, the percentage of intermediate monocytes (CD14+, CD16+) was decreased in jakinib group in comparison with RA and healthy controls [9.8 (5.1-16.4) vs 19.4 (14.4-30.3) and 20.5 (15.3-30.1), respectively; p values: < 0.001 and 0.001] (Figure 1). When comparing the subsets of T helper (Th) cells, the percentage of Th17 (CD3+CD4+CD45RA+CCR6+CXCR3-) and Th1+17 (CD3+CD4+CD45RA+CCR6+CXCR3+) were decreased in the jakinib group in comparison with healthy and RA controls (6.9 vs 9.4 and 10.0, p=0.023 and p=0.012) and (3.4, 10.4 and 8.4, p< 0.001 and p< 0.001) (Figure 2). The percentage of Effector Memory (EM, CD3+CD4+CD45RA-CD62L-) and terminally-differentiated CD45RA (TEMRA, CD3+CD4+CD45RA+CD62L-) T-helper cells were also increased in patients treated with jakinibs in comparison with healthy and RA controls [(20.5 (15.1-31.1), 10.8 (7.2-16.3), and 12.1 (7.6-17.1); p< 0.0001 and p< 0.0001)] and [1.5 (0.7-3.3), 0.4 (0.1-1.6) and 0.3 (0.1-1.2), p=0.004 and p=0.002] respectively. In addition, the percentage of Central Memory T-helper cells (CM, CD3+, CD4+,CD45RA-, CD62L+) was decreased in the jakinib group in comparison to healthy and RA controls [38.4 (43.8-45.7), 48.9 (42.1-56.5), and 48.3 (43.8-58.8), p=0.002 and p=0.001] (Figure 3). No statistically significant differences were found for naïve T-cells and regulatory T-cells.
Conclusion: The inhibition of the JAK-STAT pathway by jakinibs affects innate immune cells differently than biological DMARDs, resulting in a decrease in cytotoxic activated NK cells and intermediate monocytes, which may explain side effects such as viral infections and potential neoplasia. Additionally, JAK-STAT inhibition shifts T-helper cell subsets towards a more exhausted phenotype.
To cite this abstract in AMA style:
Lasa Teja C, Fernández-Cabero J, Comins-Boo A, San Segundo D, Lopez-Hoyos M, Blanco-Alonso R. Effects of JAK Inhibitors on Subsets of the Innate Immune System [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effects-of-jak-inhibitors-on-subsets-of-the-innate-immune-system/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-jak-inhibitors-on-subsets-of-the-innate-immune-system/