Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: Emerging Therapies (2023–2027)
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: The oral, selective Janus kinase 1 inhibitor filgotinib (FIL) significantly improved Spondyloarthritis Research Consortium of Canada (SPARCC) MRI inflammation scores (bone marrow edema) in the spine and sacroiliac joints vs placebo (PBO) in the Phase 2 TORTUGA trial (NCT03117270) in patients with active ankylosing spondylitis (AS; van der Heijde D, et al. Lancet 2018;392:2378–87). The current post hoc analysis evaluated the effects of FIL on Canada‑Denmark (CANDEN) MRI measures of spinal inflammation and structural lesions in patients from the TORTUGA trial.
Methods: TORTUGA was a PBO-controlled, multicenter, double-blind, randomized trial in which 116 patients with active AS (as per modified New York classification criteria, with sacroiliitis confirmed by central reading) were treated with FIL 200 mg (n=58) or PBO (n=58) once daily for 12 weeks. MRIs of the total spine were conducted at baseline and at the end of treatment. Scans were re‑evaluated post hoc by 2 independent experts (blinded to time point and assigned treatment) according to the detailed anatomy-based CANDEN method (Krabbe S, et al. RMD Open 2019;5:e001057); inter-reader discrepancies were resolved by an independent adjudicator. Observed changes from baseline were evaluated using analysis of covariance with factors for treatment, baseline value, and randomization stratification by prior TNF inhibitor use. Least-squares mean changes from baseline and between-group differences with 95% confidence intervals were calculated; p-values are nominal.
Results: MRI scans from 88 patients (47 FIL, 41 PBO) with an evaluable MRI at baseline and Week 12 (or early termination visit) were re-evaluated. Baseline characteristics were generally similar between subjects with and without an MRI scan. Of those with MRI scans, mean total spine inflammation score (which ranges from 0 to 614) was higher in the FIL vs PBO group and mean ankylosis score (which ranges from 0 to 460) was lower in the FIL group vs PBO at baseline (Table 1). Total spine inflammation scores decreased from baseline in the FIL group but not in the PBO group (Figure; Table 2; p=0.0003 for between-group difference). Cumulative probability plots favored FIL over PBO for change from baseline in subregion inflammation scores, including postero-lateral elements (i.e. sum of lesions in ribs, transverse processes, spinous processes, soft tissue inflammation and posterolateral vertebral body), facet joint (Figure), and vertebral body. Total spine fat lesion scores numerically increased from baseline in the FIL group but decreased in the PBO group (p=0.0878 for between-group difference; Table 2). There were no statistically significant differences between groups for changes in erosion (p=0.1956) or ankylosis (p=0.2203) scores (Table 2).
Conclusion: Compared with PBO, FIL decreased inflammation, including in the postero-lateral elements of the spine and facet joints, which has not been demonstrated previously in a PBO-controlled trial. As expected in a 12-week study period, no changes in erosion or ankylosis were seen. Due to the imbalance in MRI measures at baseline and post hoc nature of the analysis, our findings need to be confirmed in a large trial.
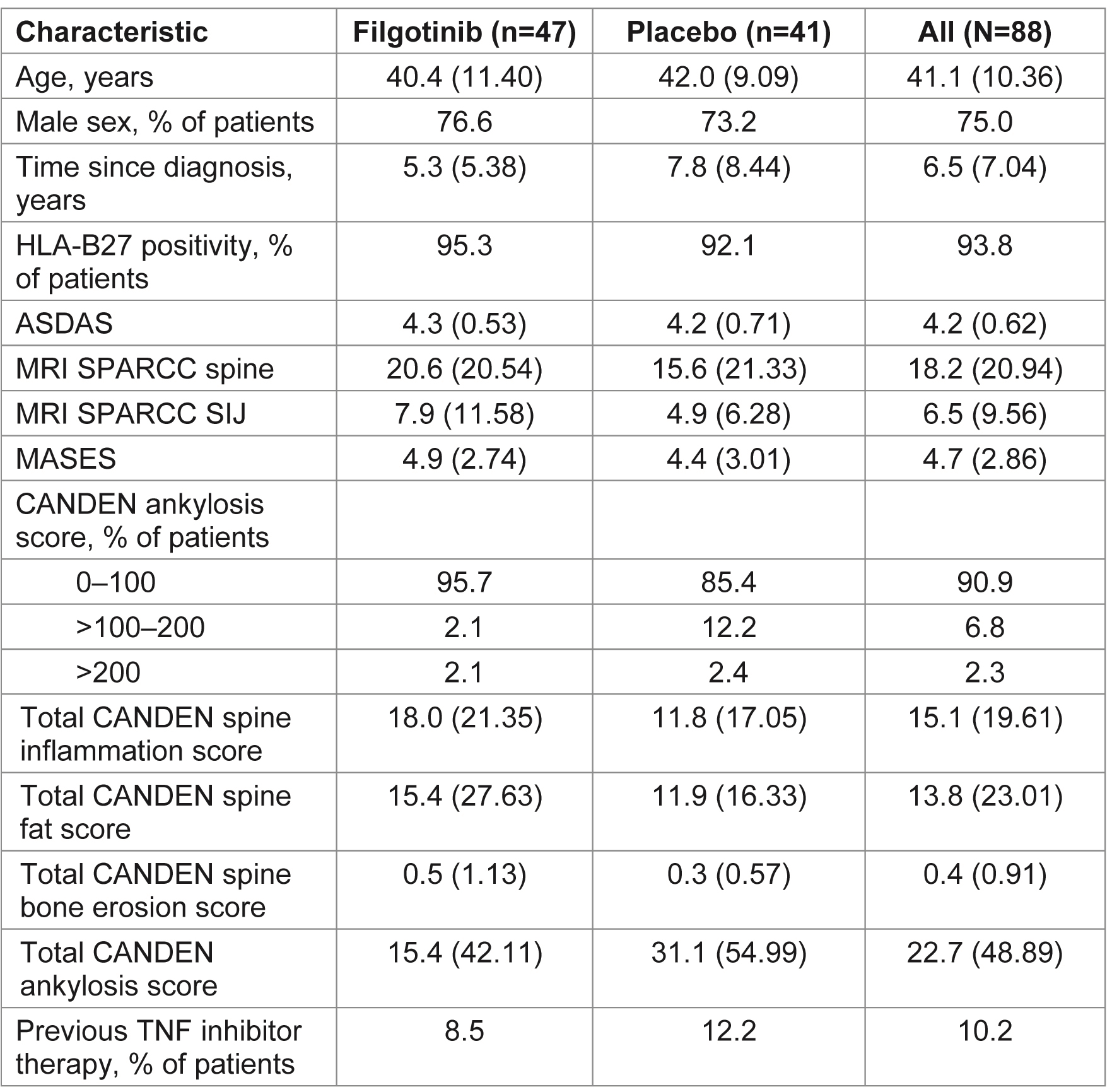 Table 1. Demographics and baseline characteristics for patients with an MRI scan. Data are mean (SD) unless otherwise indicated. ASDAS, Ankylosing Spondylitis Disease Activity Score; CANDEN, Canada-Denmark; HLA, human leukocyte antigen; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; MRI, magnetic resonance imaging; SD, standard deviation; SIJ, sacroiliac joint; SPARCC, Spondyloarthritis Research Consortium of Canada; TNF, tumor necrosis factor
Table 1. Demographics and baseline characteristics for patients with an MRI scan. Data are mean (SD) unless otherwise indicated. ASDAS, Ankylosing Spondylitis Disease Activity Score; CANDEN, Canada-Denmark; HLA, human leukocyte antigen; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; MRI, magnetic resonance imaging; SD, standard deviation; SIJ, sacroiliac joint; SPARCC, Spondyloarthritis Research Consortium of Canada; TNF, tumor necrosis factor
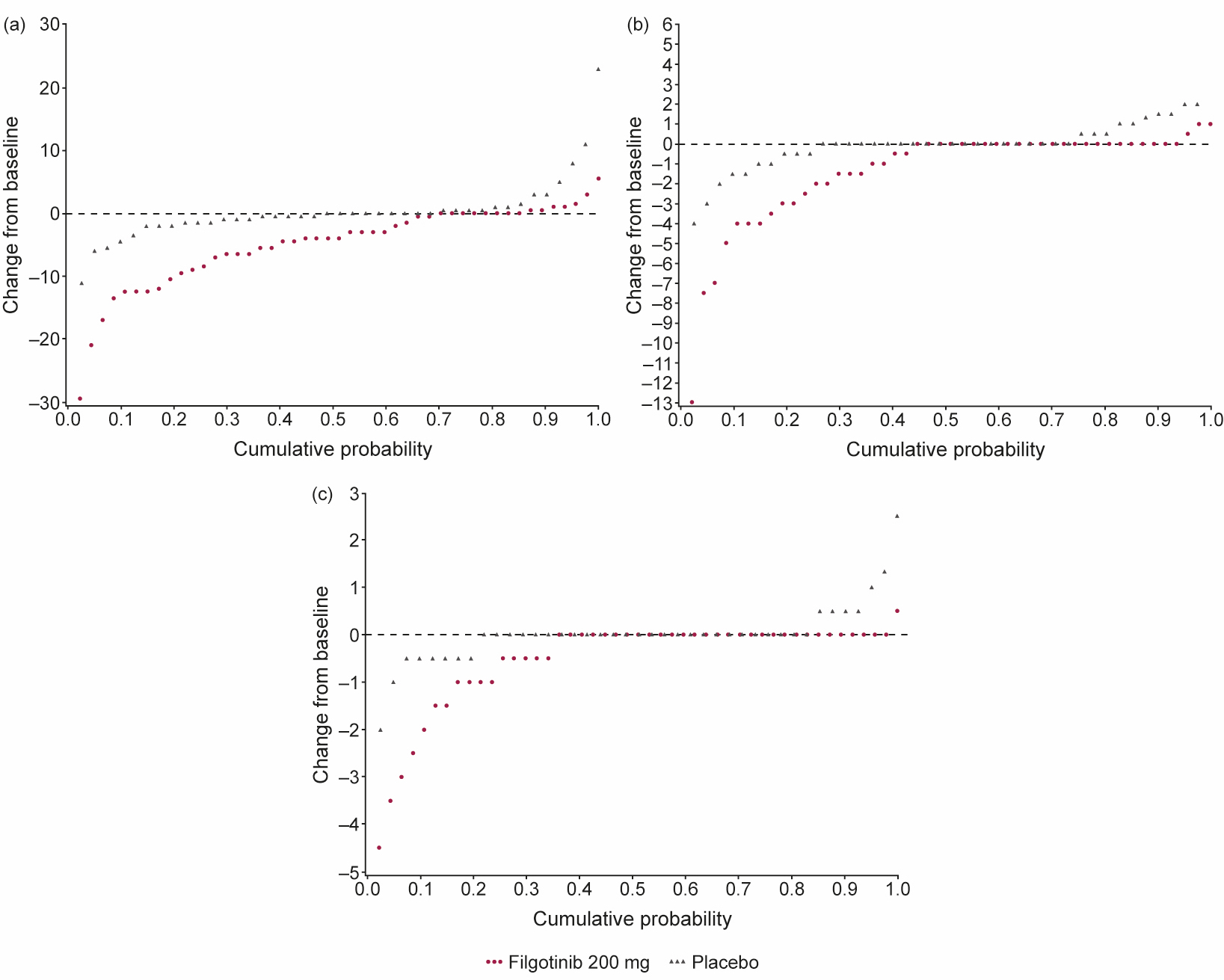 Figure 1. Change from baseline in (a) total CANDEN spine inflammation score, (b) posterior elements inflammation subregion score, and (c) facet joint inflammation subregion score. CANDEN, Canada-Denmark
Figure 1. Change from baseline in (a) total CANDEN spine inflammation score, (b) posterior elements inflammation subregion score, and (c) facet joint inflammation subregion score. CANDEN, Canada-Denmark
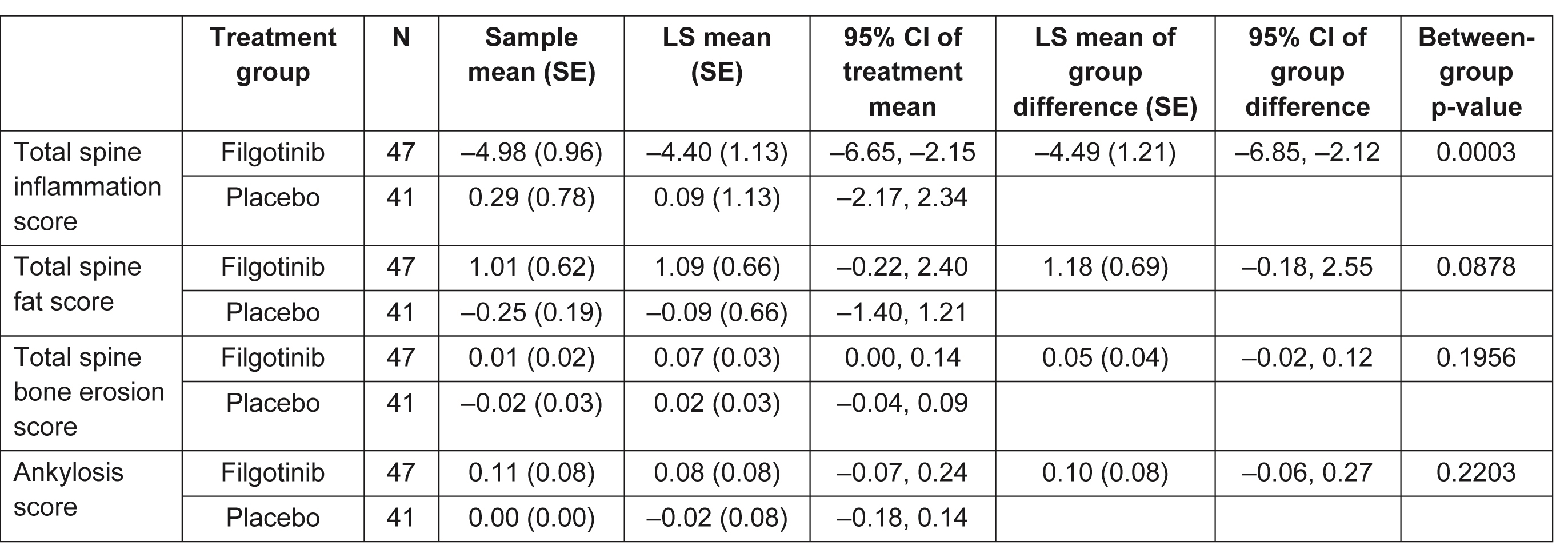 Table 2. Change from baseline at Week 12 in CANDEN total spine inflammation, total spine fat, total spine bone erosion, and ankyloses scores. CANDEN, Canada-Denmark; CI, confidence interval; LS, least squares; SE, standard error
Table 2. Change from baseline at Week 12 in CANDEN total spine inflammation, total spine fat, total spine bone erosion, and ankyloses scores. CANDEN, Canada-Denmark; CI, confidence interval; LS, least squares; SE, standard error
To cite this abstract in AMA style:
Maksymowych W, Østergaard M, Landewé R, Barchuk W, Liu K, Tasset C, Gilles L, Hendrikx T, Besuyen R, Baraliakos X. Effects of Filgotinib on Spinal Lesions in Patients with Ankylosing Spondylitis: Magnetic Resonance Imaging Data from the Placebo-Controlled, Double‑Blind, Randomized TORTUGA Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effects-of-filgotinib-on-spinal-lesions-in-patients-with-ankylosing-spondylitis-magnetic-resonance-imaging-data-from-the-placebo-controlled-double%e2%80%91blind-randomized-tortuga-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-filgotinib-on-spinal-lesions-in-patients-with-ankylosing-spondylitis-magnetic-resonance-imaging-data-from-the-placebo-controlled-double%e2%80%91blind-randomized-tortuga-trial/
