Session Information
Date: Wednesday, November 13, 2019
Title: 6W011: RA – Diagnosis, Manifestations, & Outcomes V: Treatment (2870–2875)
Session Type: ACR Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Anemia, thrombocytopenia and leukopenia in RA patients treated with non-Janus Kinase 1 (JAK1) selective inhibitors may be due to inhibition of hematopoietic growth factors signaling through JAK2. Therefore, we investigated the extent of anemia, thrombocytopenia and leukopenia in patients with active RA with a prior inadequate response/intolerance to biological DMARD (bDMARD) treated with filgotinib (FIL), a novel and selective JAK1 inhibitor, during a Phase 3, 24-week trial (FINCH-2; NCT02873936).
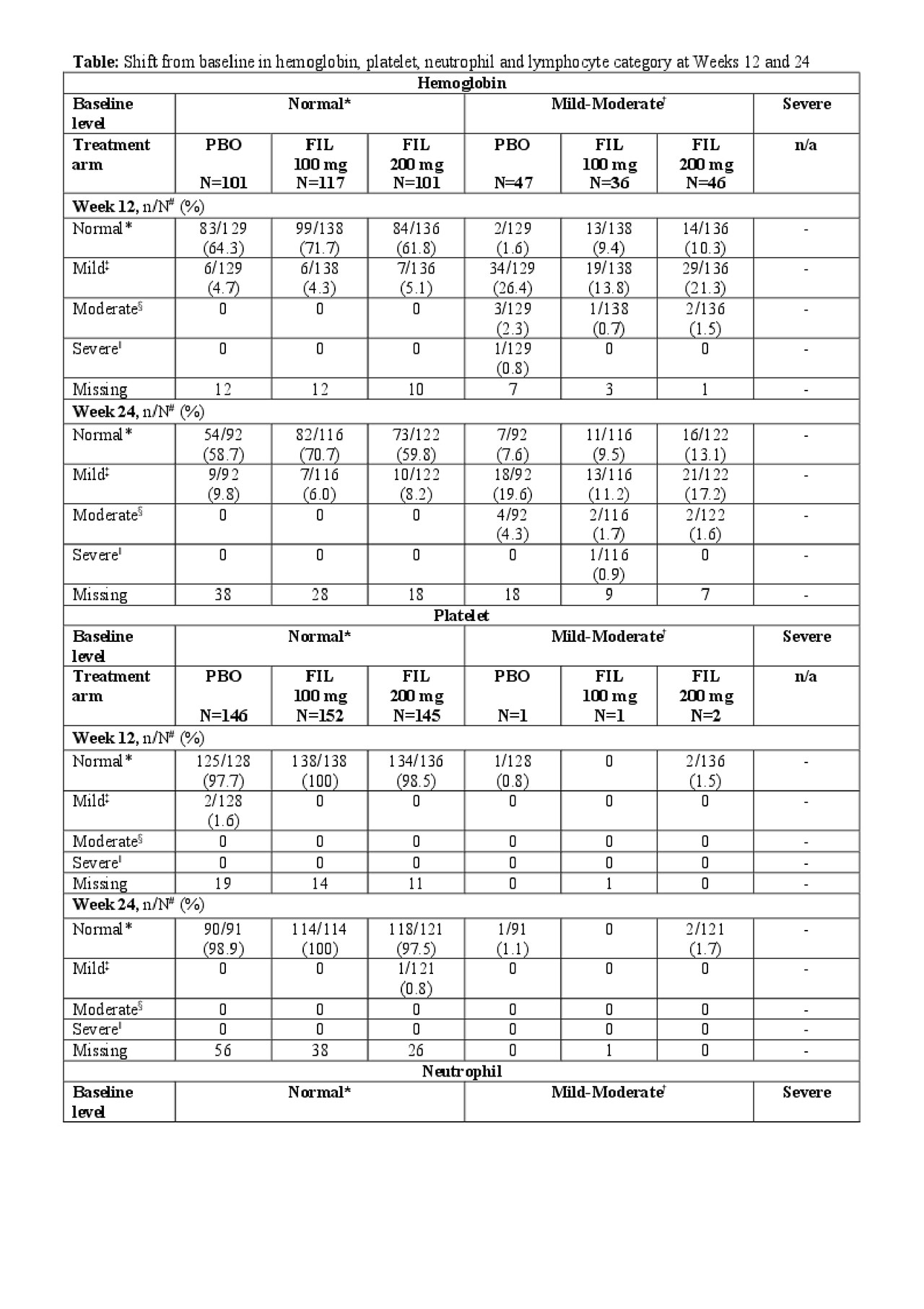
Methods: In the randomized, double-blind, placebo-controlled Phase 3 FINCH-2 trial, patients were randomized 1:1:1 to receive oral FIL 200 mg, 100 mg, or placebo (PBO) once daily for 24 weeks in addition to conventional synthetic DMARDs. We assessed shifts from baseline at 12 and 24 weeks in hemoglobin, platelets, neutrophils and lymphocytes in FINCH-2 patients, assessed by baseline values as normal, mild-moderate and severe for these variables.
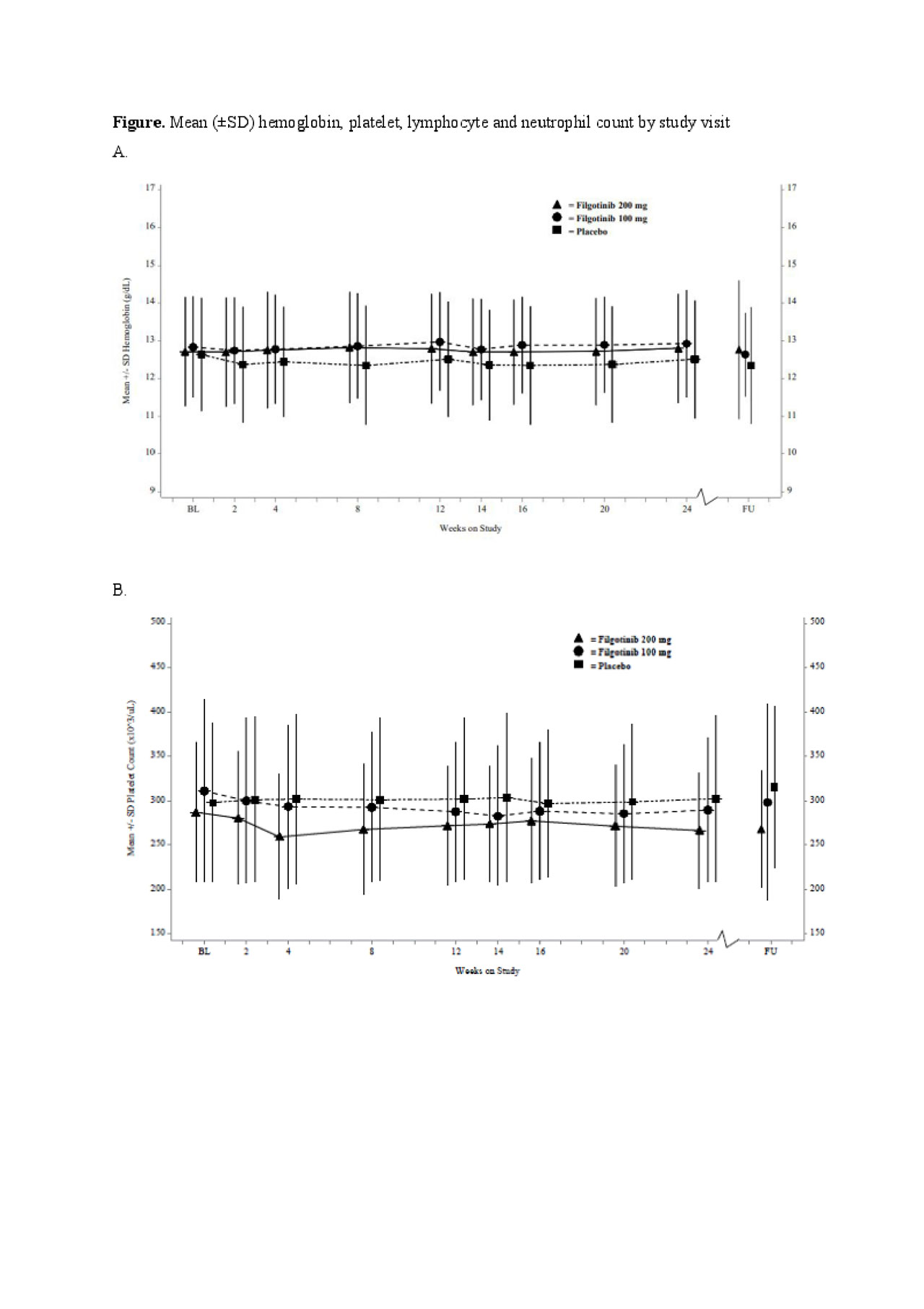
Results: A total of 448 patients were enrolled and treated, FIL 200 mg, n = 147; FIL 100 mg, n = 153; PBO, n = 148. Overall, hemoglobin levels, platelet, lymphocyte and neutrophil counts remained consistent throughout the study (Figure). At baseline, 129 (28.8%), 4 (0.9%), 10 (2.2%) and 26 (5.8%) patients had mild-moderate low levels of hemoglobin, platelet, neutrophil and lymphocyte, respectively, and 5 (1.1%) had severely low levels of lymphocytes. Of the patients with mild-moderate hemoglobin levels at baseline, 13.1% with FIL 200 mg, 9.5% with FIL 100 mg, and 7.6% with PBO achieved normal hemoglobin at Week 24, respectively (Table). Of those with normal baseline hemoglobin levels, only 6.0–9.8% had mild low levels at Week 24. All patients with baseline mild-moderate low platelets and neutrophils had normal levels at Week 24, except for one patient with mild neutropenia receiving FIL 100 mg. Of the patients with normal platelet and neutrophil levels at baseline, >94% maintained these at Week 24 in all treatment groups. By Week 24, 3.2%, -5.2% and 2.2% of patients treated with FIL 200 mg, FIL 100 mg and PBO, respectively in the mild-moderate subgroup and 1.7% in the severe subgroup treated with FIL 100 mg had normal lymphocyte counts.
Conclusion: In this FINCH-2 subgroup analysis, most patients with normal hemoglobin, platelet, lymphocyte and neutrophil levels at baseline maintained them over 24 weeks of FIL treatment. Of the patients with mild-moderately low hemoglobin at baseline, >9% shifted towards hemoglobin normalization. Similar patterns of improvement from baseline were observed for platelet, lymphocyte and neutrophil counts. These results suggest that FIL does not increase the incidence of anemia, thrombocytopenia or leukopenia in patients who entered the study with active RA despite prior biologic therapies.
To cite this abstract in AMA style:
Genovese M, Kalunian K, Gottenberg J, Bartok B, Tan Y, Guo Y, Tasset C, Sundy J, de Vlam K, Walker D, Takeuchi T. Effects of Filgotinib on Anemia, Thrombocytopenia and Leukopenia: Results from a Phase 3 Study in Patients with Active Rheumatoid Arthritis and Prior Inadequate Response or Intolerance to Biological DMARDs [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effects-of-filgotinib-on-anemia-thrombocytopenia-and-leukopenia-results-from-a-phase-3-study-in-patients-with-active-rheumatoid-arthritis-and-prior-inadequate-response-or-intolerance-to-biological-d/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-filgotinib-on-anemia-thrombocytopenia-and-leukopenia-results-from-a-phase-3-study-in-patients-with-active-rheumatoid-arthritis-and-prior-inadequate-response-or-intolerance-to-biological-d/