Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor approved in moderate to severe plaque psoriasis, has demonstrated significant improvements in cutaneous manifestations in the phase 2 PAISLEY SLE study (NCT03252587). TYK2, an important mediator of cytokine signaling (eg, types I and III IFNs, IL-23, and IL-12) involved in immune-specific responses, plays a role in cutaneous lupus erythematosus (CLE) pathophysiology. In the phase 2 PAISLEY CLE study (NCT04857034), the primary endpoint of Cutaneous Lupus Erythematosus Disease Area and Severity Index activity (CLASI-A) improvement was met with deucravacitinib in patients with CLE. This post hoc analysis aimed to identify dysregulated gene modules associated with CLE, evaluate the impact of deucravacitinib on these modules, and determine their predictive value for response to deucravacitinib.
Methods: Gene expression in whole blood was evaluated using a targeted PCR array (DxTerity AIP panel) in samples from patients with CLE with or without SLE treated in PAISLEY CLE (deucravacitinib 3 mg [n = 24] or 6 mg twice daily [n = 22] vs placebo [PBO; n = 24]). Selected genes were normalized and clustered into predefined modules, including IFN-5 (5-gene module associated with type I IFN transcription; MX1, HERC5, IFIT1, RSAD2, EIF2AK2), IFN-γ (TRIM38, SP100, LBA1), and inflammation (CXCL10, LGALS3BP, APOL6, TNFSF13B). Gene module expression (GME) in baseline (BL) samples from patients adjusted for covariates and modeled by least-squares mean change was compared to GME in age- and sex-matched normal healthy volunteers (NHVs). Changes in GME with deucravacitinib vs PBO were compared using mixed model for repeat measures. Correlation analyses of clinical response with GME at BL and change from BL were performed using Spearman’s rank correlation.
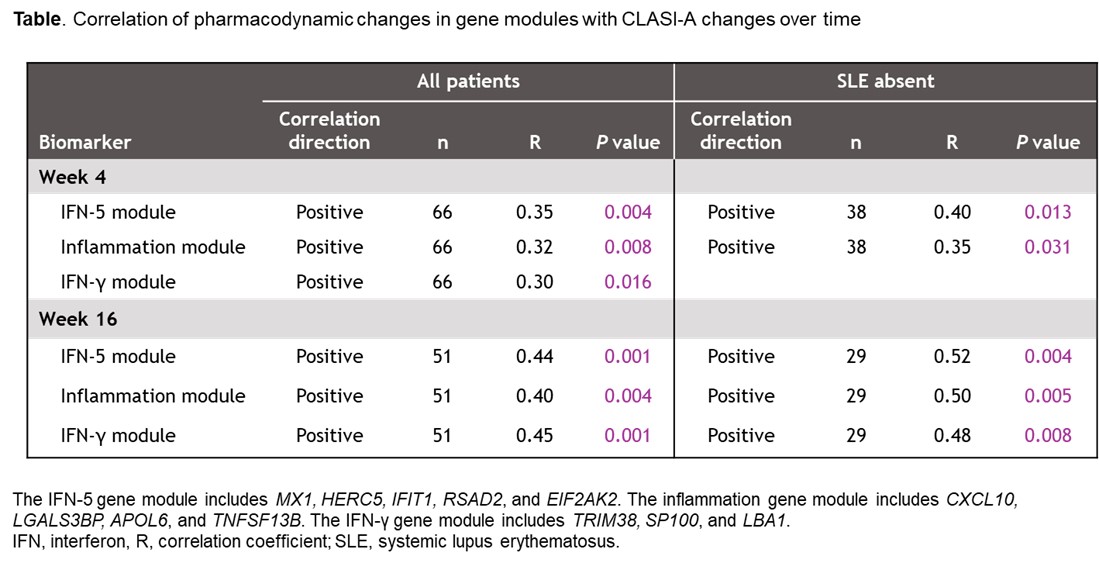
Results: Higher BL CLASI-A was associated with higher BL IFN-5 GME (P = 0.0005). There were no significant differences in BL CLASI-A or the ratio of high-to-low IFN-5 GME by SLE subgroup. IFN-5, IFN-γ, and inflammation GME were significantly elevated at BL in patients vs NHVs in both SLE subgroups (P = 0.0001 [IFN-γ, SLE absent]; P < 0.0001 [all others]). Higher BL IFN-5 and inflammation GME correlated with more severe BL CLASI-A (P = 0.002−0.007). Deucravacitinib significantly reduced IFN-5, IFN-γ, and inflammation GME vs PBO, normalizing to NHV levels (Figure); changes correlated with improved CLASI-A, and correlations strengthened over time (Table). BL IFN-γ GME correlated with response to deucravacitinib at W4 (P = 0.044) and W16 (P = 0.009); in patients with SLE, BL IFN-5 GME correlated with response to deucravacitinib at W4 (P = 0.025). Responders with a ≥ 50% reduction from baseline in CLASI-A had greater decreases in IFN-5 (P = 0.0051) and IFN-γ (P = 0.0505) GME vs nonresponders.
Conclusion: IFN and inflammation gene modules were dysregulated in CLE and positively correlated with CLASI-A. Normalization of these signatures with deucravacitinib correlated with CLASI-A improvement. Results warrant further evaluation of deucravacitinib and the utility of these biomarkers in patients with cutaneous manifestations of lupus erythematosus.
To cite this abstract in AMA style:
Kahlenberg J, Werth V, Wenzel J, Schwarz J, Liu J, Johnson B. Effects of Deucravacitinib on Dysregulated Autoimmune Gene Modules That Correlate With Skin Disease Severity and Treatment Response in Patients With Cutaneous Manifestations of Lupus Erythematosus: Biomarker Results From a Global, Randomized, Placebo-Controlled Phase 2 Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effects-of-deucravacitinib-on-dysregulated-autoimmune-gene-modules-that-correlate-with-skin-disease-severity-and-treatment-response-in-patients-with-cutaneous-manifestations-of-lupus-erythematosus-bi/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-deucravacitinib-on-dysregulated-autoimmune-gene-modules-that-correlate-with-skin-disease-severity-and-treatment-response-in-patients-with-cutaneous-manifestations-of-lupus-erythematosus-bi/

.jpg)