Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: To conduct secondary endpoint analyses of the effects of subcutaneously-administered blisibimod (A-623, AMG 623), an inhibitor of B-cell activating factor (BAFF), on patient-reported outcomes and indices of disease activity in patients with systemic lupus erythematosus (SLE) during the phase 2b clinical trial PEARL-SC (NCT01162681).
Methods: 547 SLE patients who met the ACR classification criteria, and had anti-double-stranded DNA or anti-nuclear antibodies, and SELENA-SLEDAI score ≥6 at baseline, were enrolled into the PEARL-SC study, and randomized 1:1 to receive placebo or blisibimod administered at 1 of 3 dose levels, 100 mg weekly (QW), 200 mg QW, or 200 mg every 4 weeks for up to 52 weeks (with a median of 37 weeks) or until the last subject completed 6 months of study drug therapy. Patient self-reported outcomes were evaluated using the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale, and disease activity evaluated within SELENA-SLEDAI organ domains.
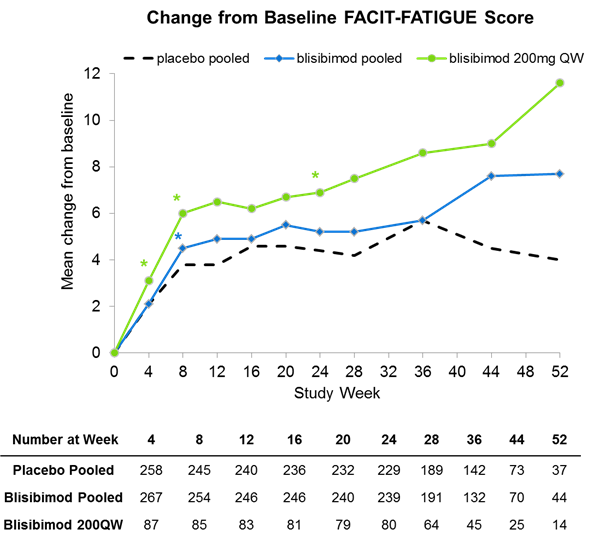
Results: Significant improvements in measures of disease activity, including the SLE responder Index-8 (SRI-8) in subjects with severe baseline disease (defined at SELENA-SLEDAI score≥10 and receiving steroids), especially at the highest blisibimod dose of 200mg QW were reported previously (Furie et al. 2014). Approximately 76% of subjects had SELENA-SLEDAI musculoskeletal organ involvement at enrollment, and 89% of subjects had mucocutaneous organ involvement. At Week 24, approximately 12% and 39% of subjects randomized to the 200mg QW blisibimod arm had musculoskeletal or mucocutaneous organ involvement, compared with approximately 15% and 42% respectively in the placebo arm (p<0.2 to p<0.05 across manifestations evaluated over Weeks 12 through 24). A concomitant tendency toward improved self-reported fatigue was observed amongst subjects randomized to blisibimod based on the FACIT-Fatigue scale, especially in the 200mg QW group (N=80) where a mean 6.9-point improvement from baseline was reported at Week 24 (p=0.065) compared with 4.4 with placebo (N=229). Based on exploratory statistical analyses, the effects of blisibimod on FACIT-fatigue were significantly better than placebo (p<0.05) as early as Week 8.
Blisibimod was safe and well-tolerated at all dose levels with no meaningful imbalances in serious adverse events or infections between blisibimod and placebo. Amongst the commonly-reported AEs, imbalance was observed only with injection site reactions (200mg QW blisibimod=15%, matched placebo=7%), but these were not serious or severe.
Conclusion: Fatigue remains a debilitating manifestation of lupus. In this trial, blisibimod showed a tendency toward improved mucocutaneous and musculoskeletal disease activity as well as patient self-reported fatigue. These data support further evaluation of blisibimod in patients with SLE.
Disclosure:
M. Petri,
Anthera Pharmaceuticals Inc,
5,
Anthera Pharmaceuticals Inc,
9;
R. S. Martin,
Anthera Pharmceuticals Inc,
1,
Anthera Pharmaceuticals Inc,
3;
C. Hislop,
Antehra Pharmaceuticals Inc,
1,
Anthera Pharmaceuticals Inc,
3;
M. A. Scheinberg,
Anthera Pharmaceuticals Inc,
9;
R. Furie,
Anthera Pharmaceuticals Inc,
5,
Anthera Pharmaceuticals Inc,
9.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-blisibimod-an-inhibitor-of-b-cell-activating-factor-on-patient-reported-outcomes-and-disease-activity-in-patients-with-systemic-lupus-erythematosus/