Session Information
Date: Monday, November 11, 2019
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Background: In a randomized controlled trial of abatacept in early diffuse systemic sclerosis (Khanna D, et al ACR 2018), we observed early clinical flares (< 2 months into treatment with study drug) in 5 of 88 patients, including 4 renal crisis and one early pulmonary deterioration. Four of these patients (3 renal crisis and pulmonary decline) were receiving abatacept. Early depletion of regulatory T cells (Tregs) was recently observed in a study of abatacept for multiple sclerosis (Glatigny et al, J Immunol, 2019:1373-82). We asked whether depletion of Tregs occurred early in SSc patients treated with abatacept, and whether this could be linked to concurrent clinical deterioration.
Methods: Methods: We used multi-parameter flow cytometry to enumerate Tregs and other lymphocyte subsets, such as T peripheral helper cells (Tph) at times 0, 1, 3 and 6 months of the ASSET study. We stratified patients, according to their pattern of gene expression on baseline skin biopsies, into three predefined distinct subgroups, termed inflammatory, fibroproliferative and normal-like.
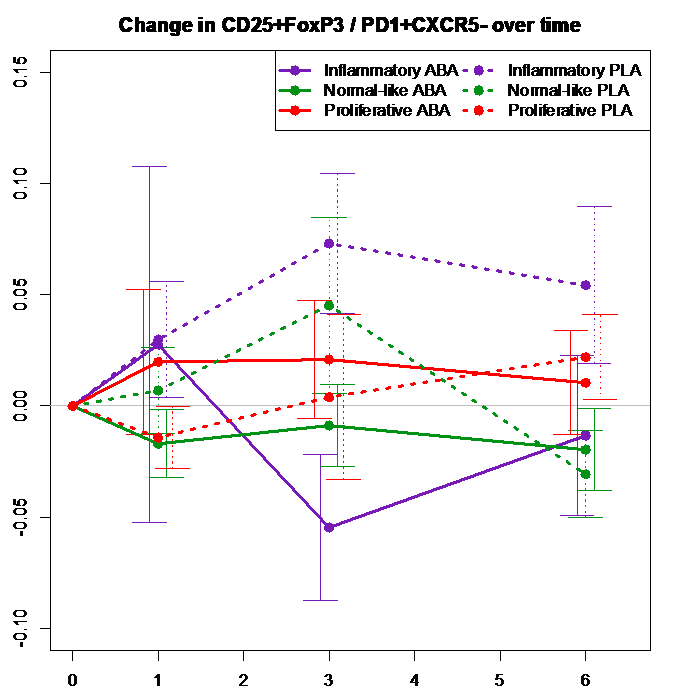
Results: Results: The SSc patients who experienced serious early clinical deterioration were all in the inflammatory gene expression subgroup. The numbers of Tregs did not differ at baseline comparing SSc patients (4.7% of CD4+) versus controls (4.14%, p = 0.235), and was not lower in the inflammatory subgroup at baseline (5%), compared with fibroproliferative (4.29%) or normal-like (4.78%) subgroups (p values not significant comparing these subgroups to each other). With abatacept treatment the Treg number progressively fell in the inflammatory subgroup from month 0 to 3 (p=0.02 at 1 month, 0.0009 at 3 months, 0.02 at 6 months). At 3 months placebo-treated inflammatory subgroup patients had a mean 6.42% Tregs within CD4+ cells compared to 2.49% in abatacept-treated patients. In the normal-like group there was a Treg reduction in the abatacept-treated patients at 3 months (p=0.03). In the fibro-proliferative group Tregs rose in both placebo and abatacept-treated patients (p not significant). We also noticed that changes of Tregs and Tph cells tended to correlate negatively with each other in the inflammatory group patients early during abatacept treatment (r = -0.38, month 0 to 1), compared to patients who received placebo (r = +0.52) and those who received abatacept in the other 2 gene expression subgroups. We created a novel parameter, the ratio of Treg/Tph cells, to further assess immune cell imbalance that might occur with abatacept treatment in early SSc. This ratio declined sharply early during abatacept treatment (p = 0.007 at 3 months)(Figure 1), and the change was primarily driven by patients in the inflammatory subgroup.
Conclusion: Conclusion: Stratification of SSc patients into predefined subgroups based on skin biopsy gene expression predicts which patients are at risk for early flares on abatacept. A proposed mechanism for such flares involves Treg depletion and early Treg/Teffector cell imbalance. Although clinical benefit appears in these patients by 6-12 months, initial treatment may require agents other than or in addition to abatacept. This needs to be further confirmed in other ongoing trials of abatacept.
To cite this abstract in AMA style:
Fox D, Whitfield M, Berrocal V, Lundy S, Campbell P, Rasmussen S, Ohara R, Stinson A, Wiewiora E, Spino C, Bush E, Furst D, Pillai S, Khanna D. Effects of Abatacept on T Regulatory Cells in Early Diffuse Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effects-of-abatacept-on-t-regulatory-cells-in-early-diffuse-systemic-sclerosis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-abatacept-on-t-regulatory-cells-in-early-diffuse-systemic-sclerosis/