Session Information
Date: Sunday, November 8, 2020
Title: Epidemiology & Public Health Poster III: Inflammatory Rheumatic Disease
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an orally applied Janus kinase inhibitor, which is approved for rheumatoid arthritis (RA) treatment in the USA since 2012, so that ‘real world data’ are already available there for many years. The prospective study ESCALATE-RA is the first non-interventional study with tofacitinib in Germany (ClinicalTrials.gov; NCT03387423). The first patient was enrolled in November 2017, the year of tofacitinib EMA-approval.
Methods: This 5-year study will enroll about 1500 patients at 200 centers in Germany. Adult patients eligible for tofacitinib therapy are documented in a standardized manner from the first tofacitinib intake for 24 months. According to standard clinical practice, patient documentation is expected quarterly and includes data on demographics, effectiveness, adverse events (AEs), and patient-reported outcomes. The present interim analysis (cut-off date January 31st, 2020) describes the patients in two subgroups (tofacitinib treatment without concomitant methotrexate treatment (mono-group); tofacitinib in combination with methotrexate (combi-group)), who reached the visit M12 after 12 months of treatment. In this non-interventional study, all analyses were based on observed data as reported by the site investigators with no imputation for missing data. Since this is an ongoing study, small changes in numbers may occur after the final data quality checks.
Results: In the M12-population (n=548), the mean age was 59 years, the majority of patients were female, had moderate to severe RA at baseline (BL), and were seropositive. 300 patients (54.7%) were treated with a bDMARD immediately prior to enrollment. 332 patients were in the mono-group and 216 patients in the combi-group at M12 (Table 1).
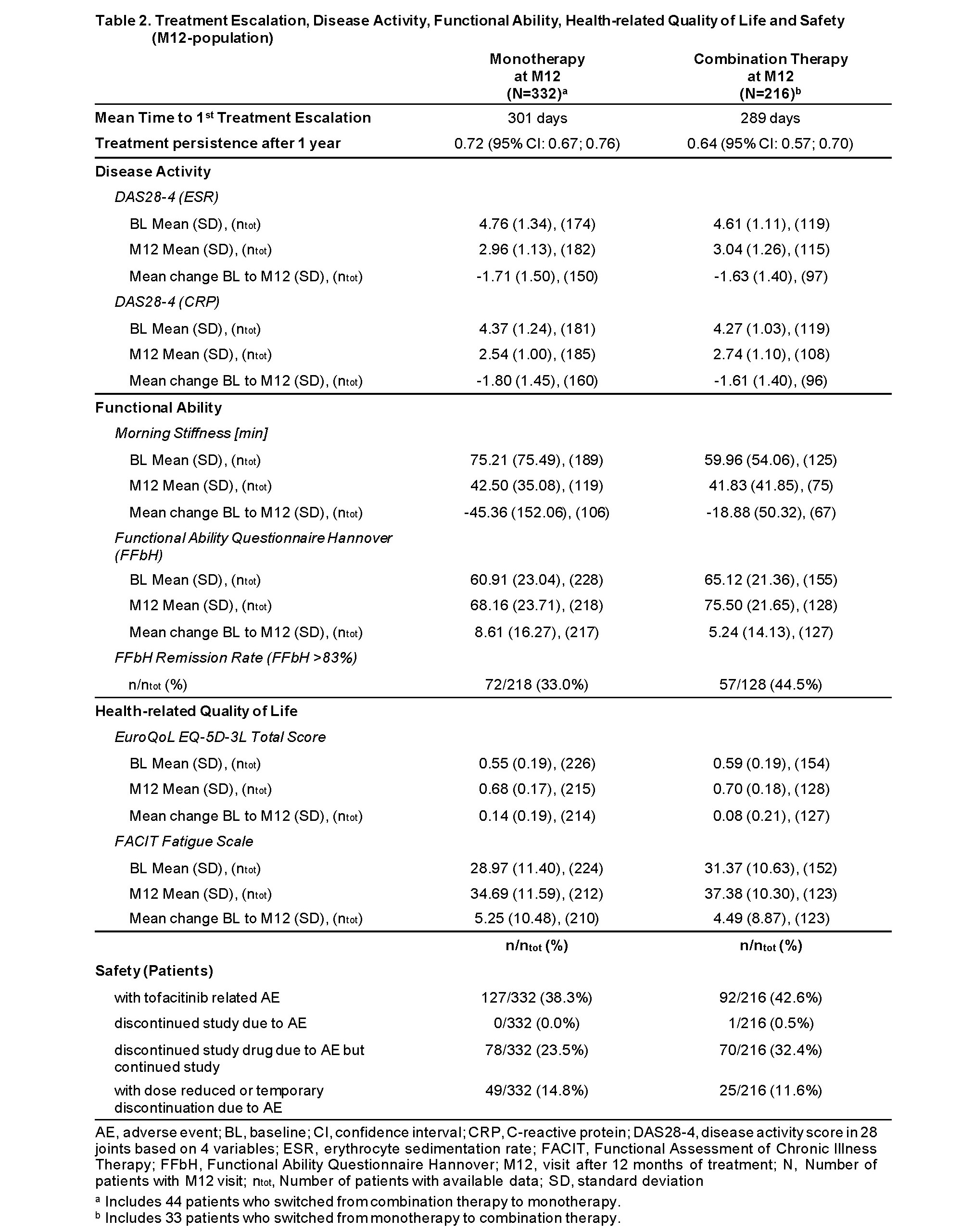
Average time to first treatment escalation was 301 days for patients with monotherapy and 289 days for patients with combination therapy. The treatment persistence after 1 year was 72% (mono-group) and 64% (combi-group) respectively. A considerable reduction of disease activity and morning stiffness was observed in both groups over time while functional ability according to FFbH (Functional Ability Questionnaire Hannover) and self-reported quality of life increased in both groups. The mean change from BL of disease activity and morning stiffness as well as functional ability was greater in mono-group compared to combi-group. Furthermore, patients in mono-group showed a greater improvement in self-reported quality of life than patients in combi-group. However, functional remission was achieved by more patients in the combi-group (44.5%) than in the mono-group (33.0%). An AE (adverse event) related to tofacitinib occurred in 38.3% of mono-group and 42.6% of combi-group (Table 2).
Conclusion: This interim analysis shows a good effectiveness profile and an increased quality of life with tofacitinib therapy. There were no unexpected AEs observed.
To cite this abstract in AMA style:
Behrens F, Prothmann U, Klopsch T, Holst A, Blindzellner L, Behmer O, Klaus P, Meng T, Löschmann P. Effectiveness, Safety and Quality of Life with Tofacitinib Treatment in Adult Patients with Rheumatoid Arthritis Under Routine Clinical Care: First Interim Results from a German Non-Interventional, Prospective, Multi-Center Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effectiveness-safety-and-quality-of-life-with-tofacitinib-treatment-in-adult-patients-with-rheumatoid-arthritis-under-routine-clinical-care-first-interim-results-from-a-german-non-interventional-pr/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-safety-and-quality-of-life-with-tofacitinib-treatment-in-adult-patients-with-rheumatoid-arthritis-under-routine-clinical-care-first-interim-results-from-a-german-non-interventional-pr/