Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: The efficacy of upadacitinib (UPA), an oral Janus kinase inhibitor (JAKi), in the treatment of rheumatoid arthritis (RA) has been demonstrated in the phase 3 SELECT clinical trial program.1–6 However, few real-world data have been reported to date. We assess the 6-month effectiveness of UPA in patients (pts) with RA initiating UPA treatment in clinical practice.
Methods: This observational study included US-based pts from the United Rheumatology Normalized Integrated Community Evidence (UR-NICE) database who initiated UPA 15 mg once daily from Aug 2019 to the data cut-off in Nov 2021. Pts with ≥6 months of baseline (BL) data before UPA initiation, and with Clinical Disease Activity Index (CDAI) score recorded at BL and 6 months (±45 days) after initiation, were included in the analysis. Effectiveness measures included CDAI score, Routine Assessment of Patient Index Data 3 (RAPID3), and Disease Activity Score in 28 joints based on C-reactive protein (DAS28-CRP); patient-reported outcomes (PROs) including 10-ADL MDHAQ (10 Activities of Daily Living Multidimensional Health Assessment Questionnaire [0-10, with higher scores indicating worse physical functioning]), Pain, and Patient’s Global Assessment of Disease Activity (PtGA); and Physician’s Global Assessment of Disease Activity (PhGA). Subgroup analyses were conducted by prior tumor necrosis factor inhibitor (TNFi) and tofacitinib (TOFA) treatment history.
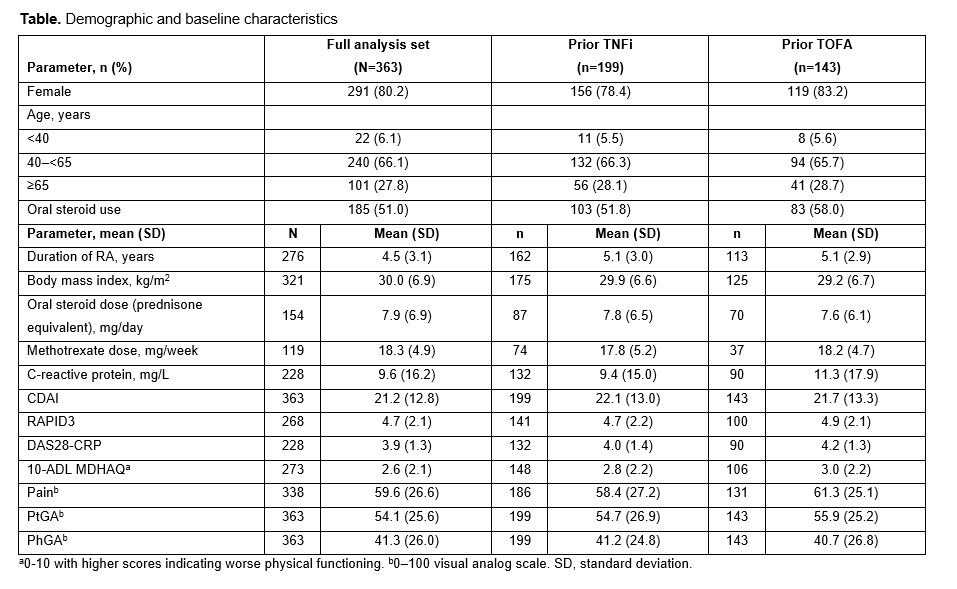
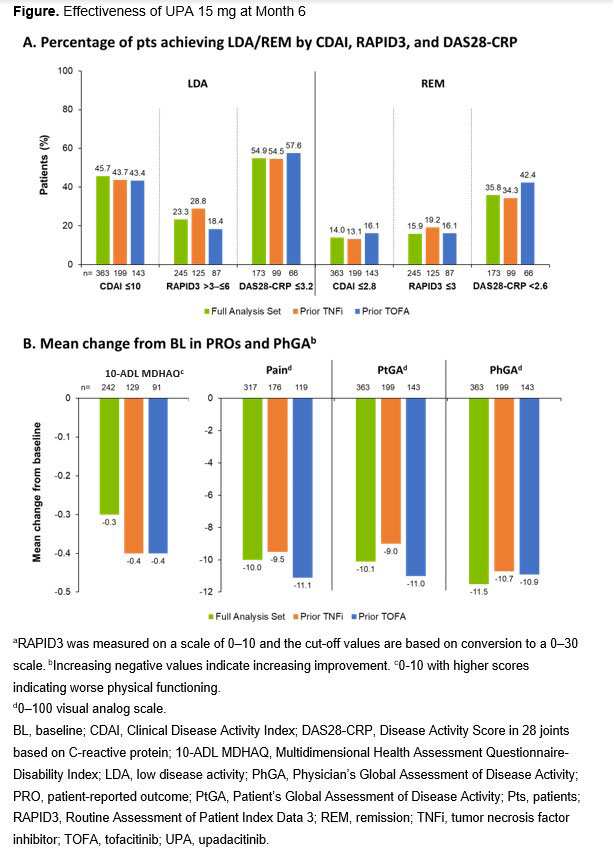
Results: 363 pts were included in the analysis and most were female (80.2%) (Table). 140 (39%) received UPA monotherapy and 223 (61%) received UPA plus conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs). 83% of pts received prior csDMARDs, 72% prior biologics (TNFi 55%), and 41% JAKis (TOFA 39%). Overall, 46% (166/363), 23% (57/245), and 55% (95/173) of pts achieved LDA by CDAI, RAPID3, and DAS28-CRP, respectively, and 14% (51/363), 16% (39/245), and 36% (62/173) of pts achieved remission (REM) by CDAI, RAPID3, and DAS28-CRP, respectively. Results were similar regardless of prior TNFi or TOFA exposure (Figure). Improvements from BL were seen in PhGA and all PROs in the total population and all subgroups.
Conclusion: In this study, almost half (46%) of pts treated with UPA achieved CDAI LDA at 6 months and 14% achieved CDAI REM. Improvements in all PROs and PhGA were observed. Effectiveness of UPA was not impacted by prior TNFi or TOFA exposure, supporting UPA as an effective treatment option in clinical practice, including in pts with prior exposure to advanced therapy.
References: 1. Burmester GR, et al. Lancet 2018;391:2503–12; 2. Smolen JS, et al. Lancet 2019;393:2303–11; 3. Fleischmann R, et al. Arthritis Rheumatol 2019;71:1788–800; 4. Genovese MC, et al. Lancet 2018;391:2513–24; 5. van Vollenhoven R, et al. Arthritis Rheumatol 2020;72:1607–20; 6. Rubbert-Roth A, et al. N Engl J Med 2020;383:1511–21.
To cite this abstract in AMA style:
Gibofsky A, Pearson M, Concoff A, Shmagel A, Zueger P, Song Y, Smith L, Wright G. Effectiveness of Upadacitinib in the Treatment of Rheumatoid Arthritis: Analysis of 6-Month Real-World Data from the United Rheumatology Normalized Integrated Community Evidence (UR-NICETM) Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-the-treatment-of-rheumatoid-arthritis-analysis-of-6-month-real-world-data-from-the-united-rheumatology-normalized-integrated-community-evidence-ur-nicetm-database/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-the-treatment-of-rheumatoid-arthritis-analysis-of-6-month-real-world-data-from-the-united-rheumatology-normalized-integrated-community-evidence-ur-nicetm-database/