Session Information
Date: Sunday, November 7, 2021
Title: Epidemiology & Public Health Poster II: Inflammatory Arthritis – RA, SpA, & Gout (0560–0593)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Our understanding of how medications such as biologic disease modifying anti-rheumatic drugs and targeted small molecules (b/tsDMARDs) influence disease activity in RA is based largely on randomized controlled trials (RCTs). However, most U.S. trials in RA are limited by small sample sizes and have often excluded patients who are older, male, racial/ethnic minorities and across the spectrum of body mass index (BMI). Whether effectiveness of b/tsDMARDs varies in real-world populations has largely been unexplored. We aimed to examine differences in longitudinal RA disease activity by demographic and clinical characteristics using the RISE registry. We simulated various treatment assignments of b/tsDMARDs that have been examined in RCTs: namely, TNF-inhibitors (TNFi) and non-TNFi.
Methods: We included 16,448 individuals from the ACR’s RISE registry with > 2 RA diagnoses (ICD-9: 714.0, ICD-10: M06.9) > 30 days apart, who had at least 2 recorded clinical disease activity index (CDAI) scores and no historical b/tsDMARD use documented in RISE. b/tsDMARD use and CDAI scores were assessed at each quarter; covariates included sex, race (white, Black, Asian, other), ethnicity (Hispanic/non-Hispanic), age, smoking, obesity (BMI > 30 mg/kg2), area deprivation index, other DMARD use, RF status, anti-CCP status, and practice type. Longitudinal targeted maximum likelihood estimation estimated the average treatment effect (ATE) of cumulative TNFi vs. non-TNFi use over a 12-month period on CDAI score among the entire population and across various subgroups based on demographic and clinical characteristics, accounting for censoring.
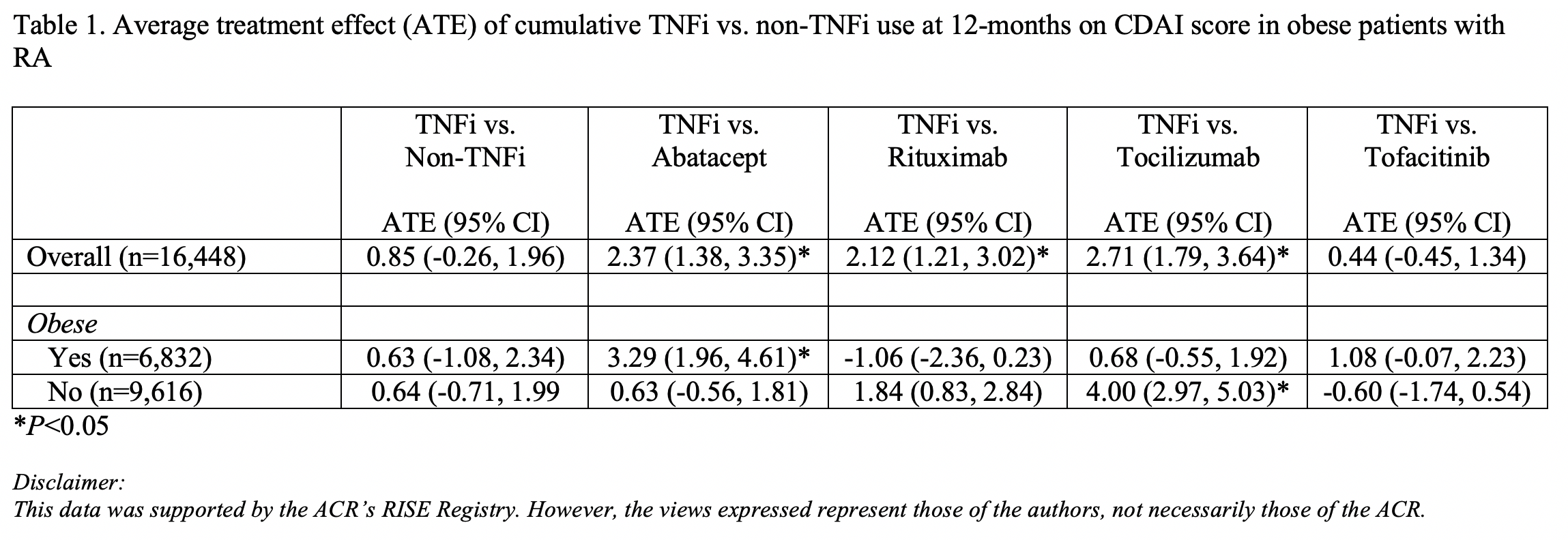
Results: Approximately 75% of patients were female with a mean age of 65.1 (+/- 13.7) years. Sixty percent of patients were white, 8% black, 2% Asian, and 30% other/mixed or unknown race; 6% were Hispanic, and 42% obese. The mean CDAI score at baseline was 11.3 (+/- 10.7). For the overall population, there was no significant difference in disease activity between TNFi and non-TNFi at 12 months (ATE= 0.85, 95% CI -0.26, 1.96; Table 1). Similarly, there was no significant difference in disease activity between TNFi and non-TNFi in obese and non-obese individuals. However, analyses by specific non-TNFi medications demonstrated higher disease activity associated with TNFi use compared to abatacept in obese patients (ATE=3.29, 95% CI 1.96, 4.61), but not non-obese patients. Contrastingly, TNFi use was associated with higher disease activity compared to tocilizumab in non-obese patients (ATE=4.00, 95% CI 2.97, 5.03), but not obese patients. In analyses comparing TNFi use and rituximab, and TNFi and tofacitinib, there were no differences in disease activity scores at 12 months for obese and non-obese patients.
Conclusion: Results from this RCT simulation study suggest that specific non-TNFi may have differential effects for obese and non-obese individuals with rheumatic disease, even though some medications are adjusted for weight. These novel findings fill gaps where RCTs have not been conducted, highlight the need for inclusion of diverse populations in future trials, and have the potential to lead to a more personalized approach to rheumatologic care.
To cite this abstract in AMA style:
Gianfrancesco M, Li J, Ja C, Seet A, Schmajuk G, Yazdany J. Effectiveness of TNFi versus Non-TNFi Biologics on Disease Activity in Obese Patients with Rheumatoid Arthritis: Data from the ACR’s RISE Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-tnfi-versus-non-tnfi-biologics-on-disease-activity-in-obese-patients-with-rheumatoid-arthritis-data-from-the-acrs-rise-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-tnfi-versus-non-tnfi-biologics-on-disease-activity-in-obese-patients-with-rheumatoid-arthritis-data-from-the-acrs-rise-registry/