Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Precapillary pulmonary hypertension (PH) in systemic sclerosis (SSc) associates with severe morbidity and mortality. The prothrombotic state observed in idiopathic pulmonary arterial hypertension (PAH) has historically supported the use of oral anticoagulants (OAC) in its management. However, the benefit of OAC in SSc-related precapillary PH remains uncertain, with studies reporting conflicting results, including possible harm. The objective of this study was to evaluate whether OAC use is associated with improved survival or reduced disease worsening in SSc patients with precapillary PH.
Methods: We conducted a cohort study using the European Scleroderma Trial and Research (EUSTAR) database to investigate the impact of OAC exposure vs. non-exposure on the prognosis of SSc patients with precapillary PH confirmed by right heart catheterization (RHC- mean PAP >20 mmHg, PVR >2 WU and PWP≤15 mmHg). Exposure to OAC was defined when lasting for at least 3 months after the RHC diagnosis.The primary outcome was a combination of all-cause death or otherwise PH worsening, the latter defined as at least one of the following: decrease of 6MWD >15%, worsening of NYHA class, onset of right heart failure, additional PAH medication, starting i.v./s.c. prostanoids, lung transplantation, or atrial septostomy. Mortality and PH worsening were also analyzed as secondary endpoints. Multivariable Cox regression was used to assess associations of exposure to OAC and the endpoints. Additionally, propensity score matching (PSM) was performed to balance confounding factors among OAC treated vs. untreated patients. Time to endpoints was assessed using Kaplan Mayer estimates. Covariates for both Cox regression and PSM were chosen based on literature support and included risk factors for worse prognosis in SSc and PH (i.e., age, sex, mPAP, reduced cardiac index, DLCO%). Missing data on the covariates were imputed using the Random Forest algorithm.
Results: Among 644 eligible patients, 173 (27%) received OAC at the time of RHC or afterwards. Patients treated with OAC were older and had more severe hemodynamic parameters (Table 1). During a median follow-up of 4 (2-6) years, 40% of patients died and 49% experienced PH worsening. Both events were recorded more frequently in the group treated with OAC, compared to non-treated patients (46% vs 37% and 59% vs 45%, respectively). Cox regression analysis did not show any significant association between OAC exposure and the combined endpoint (HR 0.996, 95% CI 0.794–1.249), mortality (HR 0.991, 95% CI 0.743–1.320) or PH worsening (HR 1.093, 95% CI 0.846–1.412) – Figure 1. PSM identified two balanced groups of 148 patients exposed and 148 not exposed to OAC. Kaplan Meier curves (Figure 2) confirmed the lack of association between OAC and the explored prognostic outcomes.
Conclusion: In this large multicenter cohort of SSc patients with precapillary PH, OAC therapy did not impact survival or disease worsening. These findings argue against the routine use of anticoagulation in this population and highlight the importance of individualized treatment decisions based on comorbidities and safety profile. Prospective studies are needed to identify whether specific subgroups may benefit from OAC therapy.
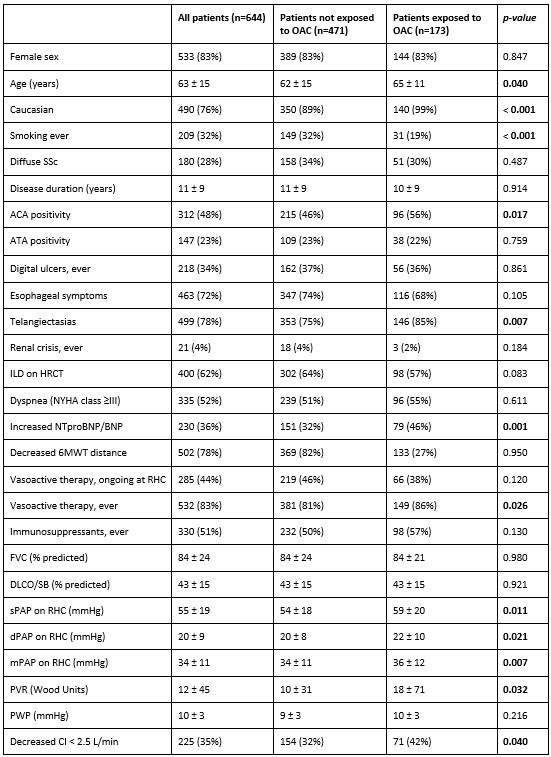 Table 1. Data about demographics, disease features, treatment exposure, functional and hemodynamic parameters, and outcome variables, compared between patients who received oral anticoagulants and those who did not. Categorial and continuous variables are reported as absolute numbers (%) and mean (standard deviation), respectively.
Table 1. Data about demographics, disease features, treatment exposure, functional and hemodynamic parameters, and outcome variables, compared between patients who received oral anticoagulants and those who did not. Categorial and continuous variables are reported as absolute numbers (%) and mean (standard deviation), respectively.
BNP, brain natriuretic peptide; CI, cardiac index; DLCO, diffusion lung capacity of carbon monoxide; HR, hazard ratio; HRCT, high resolution computed tomography; ILD; interstitial lung disease; mPAP, mean pulmonary arterial pressure; NYHA, New York Heart Association; PH, pulmonary hypertension; 6MWT, 6-minute walking test. Bold represents statistical significance with p value < 0.05.
.jpg) Figure 1. Forest plots of multivariable regression models testing the association between exposure to oral anticoagulants and the prognosis (panel a primary endpoint, panel b death, panel c pulmonary hypertension worsening) of patients with pulmonary arterial hypertension at right heart catheterization.
Figure 1. Forest plots of multivariable regression models testing the association between exposure to oral anticoagulants and the prognosis (panel a primary endpoint, panel b death, panel c pulmonary hypertension worsening) of patients with pulmonary arterial hypertension at right heart catheterization.
BNP, brain natriuretic peptide; CI, cardiac index; DLCO, diffusion lung capacity of carbon monoxide; HR, hazard ratio; HRCT, high resolution computed tomography; ILD; interstitial lung disease; mPAP, mean pulmonary arterial pressure; NYHA, New York Heart Association; PH, pulmonary hypertension; 6MWT, 6-minute walking test. Bold represents statistical significance with p value < 0.05.
.jpg) Figure 2. Survival curves for different outcome variables according to the exposure status to oral anticoagulants, in the match-control population identified using propensity score matching.
Figure 2. Survival curves for different outcome variables according to the exposure status to oral anticoagulants, in the match-control population identified using propensity score matching.
NYHA:New York Heart Association; OAC, oral anticoagulants; PH, pulmonary hypertension.
Disclosures: N. Farina: None; S. Bellando-Randone: Boehringer-Ingelheim, 2, 6; h. Bjørkekjær: Janssen, 5; D. Launay: AstraZeneca, 2, 6, BioCryst, 6, 12, travel grants, CSL Behring, 2, 6, Shire, 12, travel grants, Takeda, 2, 6; P. Carreira: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, Corbus, 2, 6, Emmerald Health Pharmaceuticals, 2, 6, Janssen, 2, 6, Mitsubishi Tanabe, 2, 6, Novartis, 2, 6, Prometheus, 2, 6; p. airò: Amgen, 12, meeting/travel support, CSL Behring, 12, meeting/travel support, Eli Lilly, 12, meeting/travel support, Janssen, 6, 12, meeting/travel support, Novartis, 6; S. Guiducci: None; D. Giuggioli: None; G. Riemekasten: AbbVie/Abbott, 6, Boehringer-Ingelheim, 2, 6, Galapagos, 6, Janssen, 2, 6, Merck/MSD, 6, Novartis, 6, Roche, 6, Sanofi, 5; c. Simeón Aznar: Boehringer-Ingelheim, 2, 6, 12, travel grants, Janssen, 2, 6, 12, travel grants, Merck/MSD, 6; C. Bergmann: None; E. Siegert: None; I. Castellví: Boehringer-Ingelheim, 2, 6, Bristol-Myers Squibb(BMS), 6, Gebro, 6, GlaxoSmithKlein(GSK), 2, Innovarderm, 2, Janssen, 2, 6, Kern Pharma, 2, 6, Novartis, 2, Roche, 6, Sanofi-Genzyme, 6; L. Saketkoo: Abbvie, 6, Argenx, 1, 2, 5, aTyr Pharmaceuticals, 12,, 1, 5, Boehringer-Ingelheim, 2, 5, 6, CSL Behring, 5, EMD Serono, 2, 5, Horizon, 5, Johnson & Johnson, 6, Kadmon, 5, Kinevant, 12,, 2, 5, Mallinckrodt, 1, 2, 5, Novartis, 1, 2, 5, Priovant, 5; J. de Vries-Bouwstra: AbbVie/Abbott, 2, 6, Boehringer-Ingelheim, 2, 6, Jannsen-Cilag, 5, Janssen, 2, 6, Roche, 5; D. Klemm: None; u. Müller-Ladner: None; A. Balbir- Gurman: None; V. Smith: Argenx, 2, Boehringer-Ingelheim, 2, 5, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 5, 6; F. Iannone: None; L. Idolazzi: None; C. Denton: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Certa Pharmaeuticals, 2, GlaxoSmithKlein(GSK), 2, Janssen, 2, Novartis, 2; e. rosato: None; B. Maurer: AbbVie/Abbott, 5, Actelion, 12, Congress support, Boehringer-Ingelheim, 1, 5, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, Medtalk, 12, Congress support, Mepha, 12, Congress support, Merck/MSD, 12, Congress support, Novartis, 2, 5, 6, Otsuka, 6, Pfizer, 12, Congress support, Protogen, 5, Roche, 12, Congress support, UCB, 12, Congress support; Y. Allanore: None; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer Ingelheim, 6, Chugai, 6, Daiichi Sankyo, 6, Eisai, 6, Eli Lilly & Co., 6, Gilead, 6, GSK, 6, IQVIA, 6, Otsuka, 6, Taisho, 6, UCB, 6; e. zanatta: None; M. Truchetet: AbbVie/Abbott, 2, 12, travel grants, Boehringer-Ingelheim, 2, Eli Lilly, 2, 6, Galapagos, 6, Merck/MSD, 6, Novartis, 6, Pfizer, 2, UCB, 2; M. Kuwana: AbbVie, 2, Asahi Kasei Pharma, 6, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, 6, Chugai, 2, 6, GlaxoSmithKlein(GSK), 2, Medical and Biological Laboratories, 5, 9, Mochida, 2, Novartis, 2, Ono Pharmaceuticals, 6; M. MARTIN: None; A. Cauli: None; K. Solanki: Apollo Hospitals Educational and Research Foundations, 12, Honorary adjunct professor; F. Del Galdo: AbbVie/Abbott, 2, 5, argenx, 2, 5, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, Calluna, 2, 5, Deepcure, 2, 5, Engitix, 2, 5, GlaxoSmithKlein(GSK), 2, 5, Janssen, 2, 5, Merck/MSD, 2, 5, Miltenyi, 2, 5, Mitsubishi-Tanabe, 2, 5, Novartis, 2, 5, Ono, 2, 5, Quell, 2, 5, RelationX, 2, 5, Serono, 2, 5, Syntara, 2, 5, Ventus, 2, 5, ZuraBio, 2, 5; A. Gheorghiu: Boehringer-Ingelheim, 6, Foundation for research in Rheumatology (FOREUM), 5; B. Anic: None; G. Kumánovics: None; G. Boleto: Boehringer-Ingelheim, 12, support for attending meetings; K. Andréasson: Janssen, 6; S. Rednic: None; L. Chung: AbbVie/Abbott, 1, Boehringer-Ingelheim, 1, CRISPR Therpeutics, 2, Cure Ventures, 2, jade, 2, Kyverna, 6, Mediar, 1, 2; s. Oliveira: None; m. cadar: None; F. Cantatore: None; C. de Souza Müller: None; V. Hsu: None; Y. Levy: None; G. Moroncini: None; J. Henes: AbbVie/Abbott, 1, 6, AstraZeneca, 1, 6, Boehringer-Ingelheim, 1, 6, Bristol-Myers Squibb(BMS), 1, 6, Eli Lilly, 1, Janssen, 1, 6, Novartis, 1, 1, 6, 6, Otsuka, 1, Pfizer, 1, Roche, 1, SOBI, 1, UCB, 1, 6; A. Balanescu: AbbVie/Abbott, 1, 6, Amgen, 2, 6, Angellini, 6, AstraZeneca, 6, Boehringer-Ingelheim, 6, Eli Lilly, 6, Ewopharma, 6, Janssen, 6, Novartis, 6, Pfizer, 6, Sandoz, 6, Sobi, 2, 6, Stada, 2, 6, Theramex, 6, UCB, 2, 6; E. De Langhe: None; C. Montecucco: None; P. Sfikakis: None; M. Iudici: Boehringer-Ingelheim, 6; S. Heimann: None; M. Vonk: Boehringer-Ingelheim: 2, 6, 15; Janssen, 2, 6, 15; Merck/MSD: 6; A. Hoffmann-Vold: AbbVie, 2, Avalyn, 2, Boehringer Ingelheim, 2, 5, 6, 15, Brostol-Myers Squibb, 2, Calluna Pharma, 2, Genentech, 2, Janssen, 2, 5, 6, Medscape, 2, 6, Merck Sharp & Dohme, 2, 6, Novartis, 6, Pliant Therapeutics, 2, Roche, 2, 6, Werfen, 2; O. Distler: 4P-Pharma, 2, 6, AbbVie/Abbott, 2, 6, Acceleron, 2, 6, Acepodia Biotech, 2, 6, Aera, 2, 6, AnaMar, 2, 6, Anaveon, 2, 6, AG, Argenx, 2, 6, AstraZeneca, 2, 6, BMS, 2, 5, 6, Calluna (Arxx), 2, 6, Cantargia AB, 2, 6, CITUS AG, 8, CSL Behring, 2, 6, EMD Serono, 2, 6, Galapagos, 2, 6, Galderma, 2, 6, Gossamer, 2, 6, Hemetron, 2, 5, 6, Innovaderm, 2, 5, 6, Janssen, 2, 6, Mediar, 2, 5, 6, mir-29 for the treatment of systemic sclerosis, 10, Mitsubishi Tanabe, 2, 5, 6, MSD Merck, 2, 6 Nkarta Inc., 2, 6, Novartis, 2, 6, Orion, 2, 6, Pilan, 2, 6, Prometheus, 2, 6, Quell, 2, 6, Sumitomo, 2, 5, 6, Topadur, 2, 5, 6, UCB, 2, 5, 6; M. Matucci-Cerinic: None; Cosimo Bruni: Beohringer Ingelheim, 2, EMDO Foundation, 5, Iten-Kohaut Foundation, 5, Scleroderma Clinical Trials Consortium (SCTC), 5, Scleroderma Research Foundation (SRF), 2
To cite this abstract in AMA style:
Farina N, Bellando-Randone S, Bjørkekjær h, Launay D, Carreira P, airò p, Guiducci S, Giuggioli D, Riemekasten G, Simeón Aznar c, Bergmann C, Siegert E, Castellví I, Saketkoo L, de Vries-Bouwstra J, Klemm D, Müller-Ladner u, Balbir- Gurman A, Smith V, Iannone F, Idolazzi L, Denton C, rosato e, Maurer B, Allanore Y, Tanaka Y, zanatta e, Truchetet M, Kuwana M, MARTIN M, Cauli A, Solanki K, Del Galdo F, Gheorghiu A, Anic B, Kumánovics G, Boleto G, Andréasson K, Rednic S, Chung L, Oliveira s, cadar m, Cantatore F, de Souza Müller C, Hsu V, Levy Y, Moroncini G, Henes J, Balanescu A, De Langhe E, Montecucco C, Sfikakis P, Iudici M, Heitmann S, Vonk M, Hoffmann-Vold A, Distler O, Matucci-Cerinic M, Bruni C. Effectiveness of Oral Anticoagulants in Precapillary Pulmonary Hypertension Associated with Systemic Sclerosis: a EUSTAR Cohort Study. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-oral-anticoagulants-in-precapillary-pulmonary-hypertension-associated-with-systemic-sclerosis-a-eustar-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-oral-anticoagulants-in-precapillary-pulmonary-hypertension-associated-with-systemic-sclerosis-a-eustar-cohort-study/
