Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor (JAKi) for the treatment of RA. CANTORAL is the first large-scale, national, observational study assessing effectiveness and safety of a JAKi (tofacitinib) in Canadian patients (pts) with RA. Here we describe primary and key secondary effectiveness and safety endpoints from the CANTORAL study. Exploratory safety analyses were also conducted in pts with baseline (BL) cardiovascular (CV) risk factors.
Methods: Pts with moderate to severe active RA who initiated tofacitinib between Nov 2017–Jul 2020 were enrolled across 45 Canadian sites and managed per clinical standard of care. Effectiveness (up to Month [M]12) was assessed in enrolled pts (full analysis set), and safety (up to M36) was evaluated in pts with ≥ 1 post-BL visit (safety analysis set) as of Feb 26, 2021. An exploratory safety analysis was also conducted in pts aged ≥ 50 yrs with ≥ 1 CV risk factor (CV+ cohort), similar to ORAL Surveillance (NCT02092467) entry criteria. Co-primary endpoints included proportions of pts achieving CDAI low disease activity (LDA; < 10) and remission (REM; < 2.8) at M6. Secondary endpoints included proportions of pts achieving CDAI LDA and REM, DAS28‑4(ESR) LDA (< 3.2) and REM (< 2.6), and DAS28‑4(CRP) < 3.2 and < 2.6, over time; proportions of pts achieving improvements in HAQ-Disability Index (HAQ-DI) ≥ minimum clinically important difference (MCID; ≥ 0.22), HAQ-DI normative values (≤ 0.25), and ≥ 50% improvement in Pain (VAS) at M3; and least squares (LS) mean change from BL (ΔBL) in tender joint count (TJC), swollen joint count (SJC), HAQ-DI, and Pain, over time. Safety outcomes included treatment-emergent adverse events (TEAEs), serious AEs (SAEs), deaths, and AEs of special interest.
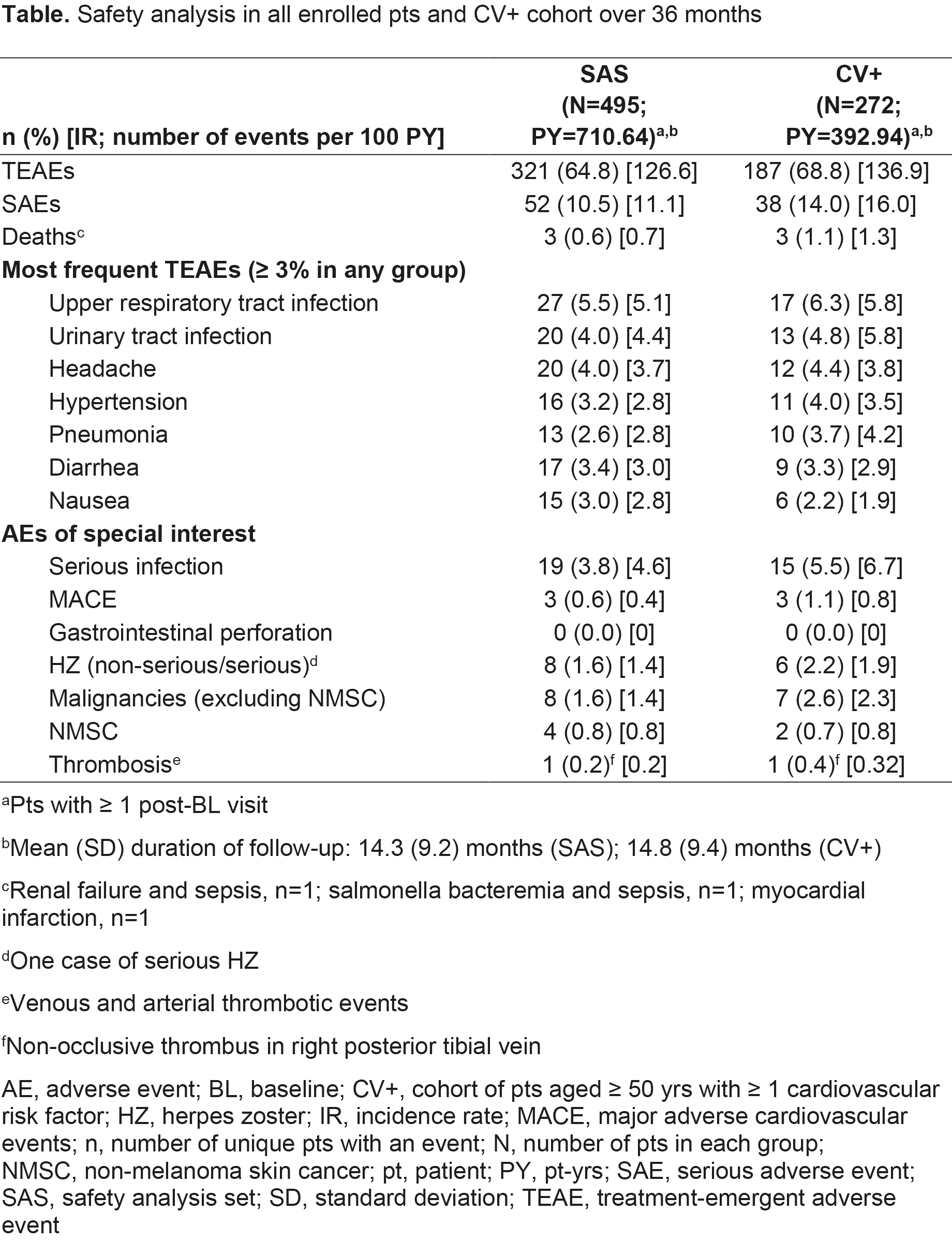
Results: 504 pts were eligible for analysis; most were female (77.8%), white (82.9%), with a mean age of 59.3 yrs and disease duration of 10.2 yrs. Most pts were biologic DMARD-naïve (66.5%), taking BL background conventional synthetic DMARDs (62.5%). At M6, 61.1% and 17.8% of pts achieved CDAI LDA and REM, respectively, with most also achieving outcomes at M3 (Figure); similar findings were seen for DAS28-4(ESR) and DAS28‑4(CRP) (Figure). LS mean ΔBL at M3 (p < 0.001) in TJC, SJC, HAQ-DI, and Pain were -5.7, -5.1, -0.3, and -20.5, respectively, wherein improvements were continued/maintained to M12. At M3, HAQ-DI MCID and normative values were achieved by 53.7% and 19.9% of pts, respectively; ≥ 50% Pain improvement was achieved by 42.9% of pts. 54.0% (n=272) of pts met inclusion criteria for the CV+ cohort, of whom 25.4% were smokers and 8.1% had a history of coronary artery disease; hypertension, diabetes mellitus, and RA-associated extra-articular features were reported in 58.8%, 17.6%, and 26.8% of CV+ pts, respectively. Most TEAEs, SAEs, deaths, and AEs of special interest were reported in CV+ pts (Table).
Conclusion: Results of CANTORAL are consistent with tofacitinib efficacy and safety in the RA clinical program, including ORAL Surveillance, and Canadian observational studies of advanced therapies. AEs were more likely to occur in pts aged ≥ 50 yrs with CV risk factors.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by T Guha, CMC Connect, funded by Pfizer Inc.
To cite this abstract in AMA style:
Haraoui B, Khraishi M, Choquette D, Lisnevskaia L, Teo M, Kinch C, Galos C, Roy P, Gruben D, Woolcott J, Vaillancourt J, Sampalis J, Keystone E. Effectiveness and Safety of Tofacitinib in Canadian Patients with RA: Primary Results from a Multicenter, Observational Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-tofacitinib-in-canadian-patients-with-ra-primary-results-from-a-multicenter-observational-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-tofacitinib-in-canadian-patients-with-ra-primary-results-from-a-multicenter-observational-study/