Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: The recombinant zoster vaccine (RZV) is US Food and Drug Administration-approved for the prevention of herpes zoster (HZ, shingles) in adults ≥50 years, and adults ≥18 years who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy. Adults with systemic lupus erythematosus (SLE) or multiple sclerosis (MS) are at increased risk of HZ and may benefit from vaccination. In this interim analysis of a retrospective cohort study, we assessed 2-dose RZV vaccine effectiveness (VE) in patients with SLE or MS, and the risk of severe SLE flare.
Methods:
Adults ≥18 years with MS or SLE were identified from 7 commercial insurers that participate in the FDA Sentinel System (including Medicare Advantage, Part C) and, separately, from Medicare (Parts A, B, and D) between 2018 and 2021-2023 (depending on data source). Using established algorithms, SLE was defined as ≥3 diagnosis codes ≥30 days apart with ≥1 diagnosis in the past year and MS as ≥3 MS-related claims (diagnosis codes or disease modifying therapy) in the past year including ≥1 diagnosis. In VE analyses, adults completing the 2-dose RZV series (≥28 days apart) were matched up to 1:4 to RZV unvaccinated comparators on SLE or MS diagnosis, insurer, sex, and age ± 5 years at the index date (i.e., 2nd RZV dose date or assigned index date for comparators); patients with HZ during the 1-year baseline were excluded. The VE outcome was first HZ (diagnosis + oral anti-viral within 7 days) ≥31 days after index. In safety analyses, adults with SLE receiving RZV dose 1 or 2 were separately matched up to 1:4 to unvaccinated patients as above, excluding those with severe SLE flare in the 90 days prior to index. The safety outcome was severe SLE flare (initiation of cyclophosphamide, rituximab, or high-dose glucocorticoids, or hospitalization for SLE or SLE-related condition) ≤90 days after index. Adjusted hazard ratios (HR) and VE (1-adjusted HR*100) were estimated using Cox models with propensity score-based inverse probability of treatment weights to balance confounders.
Results:
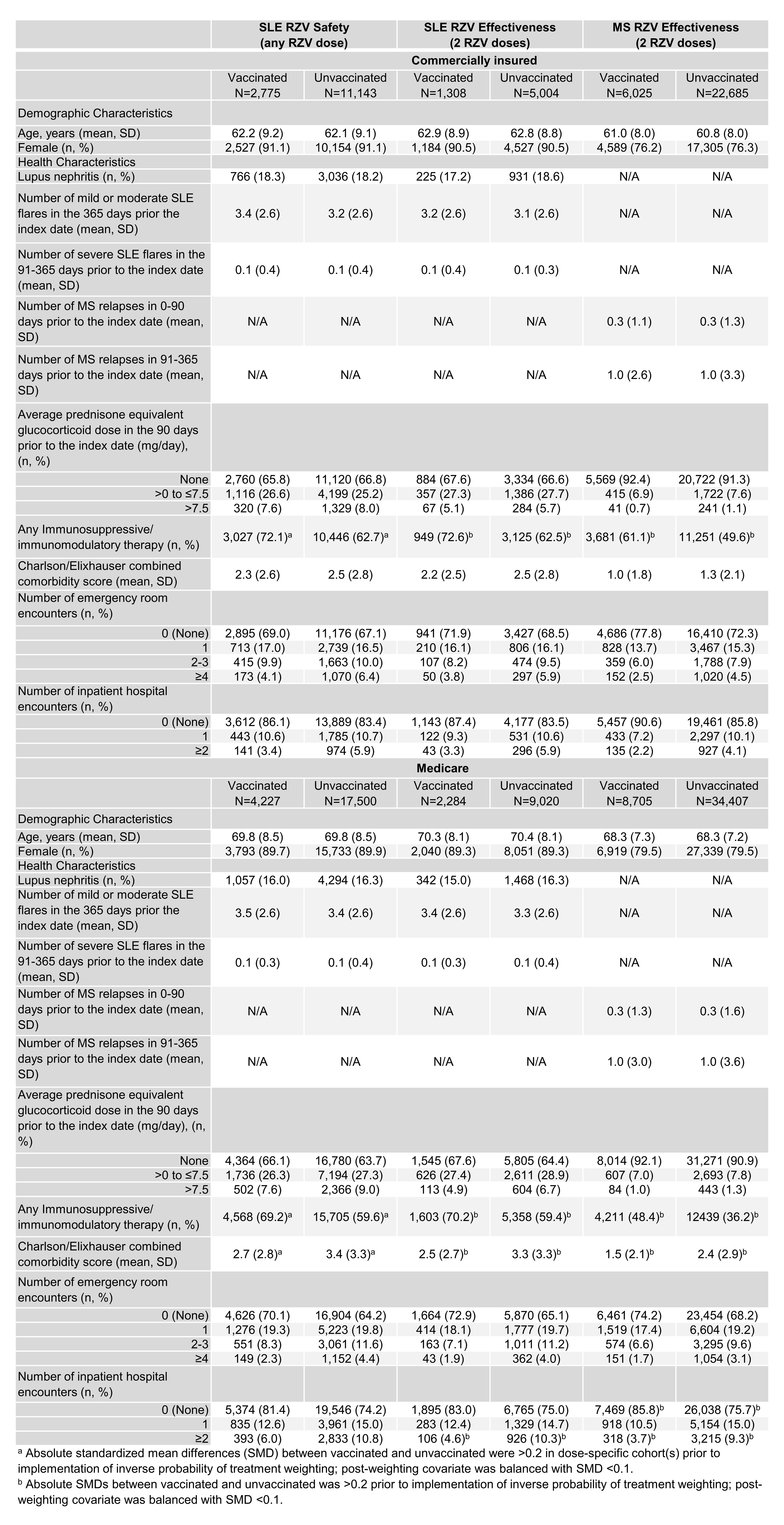
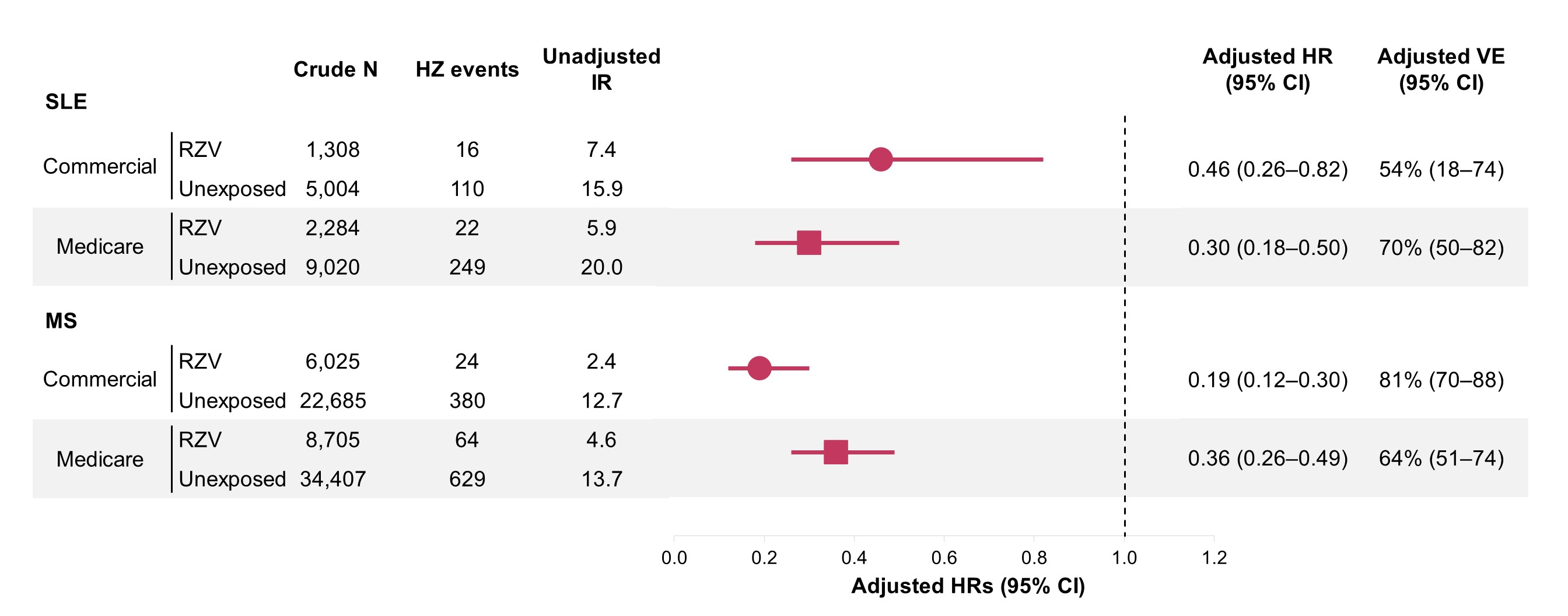
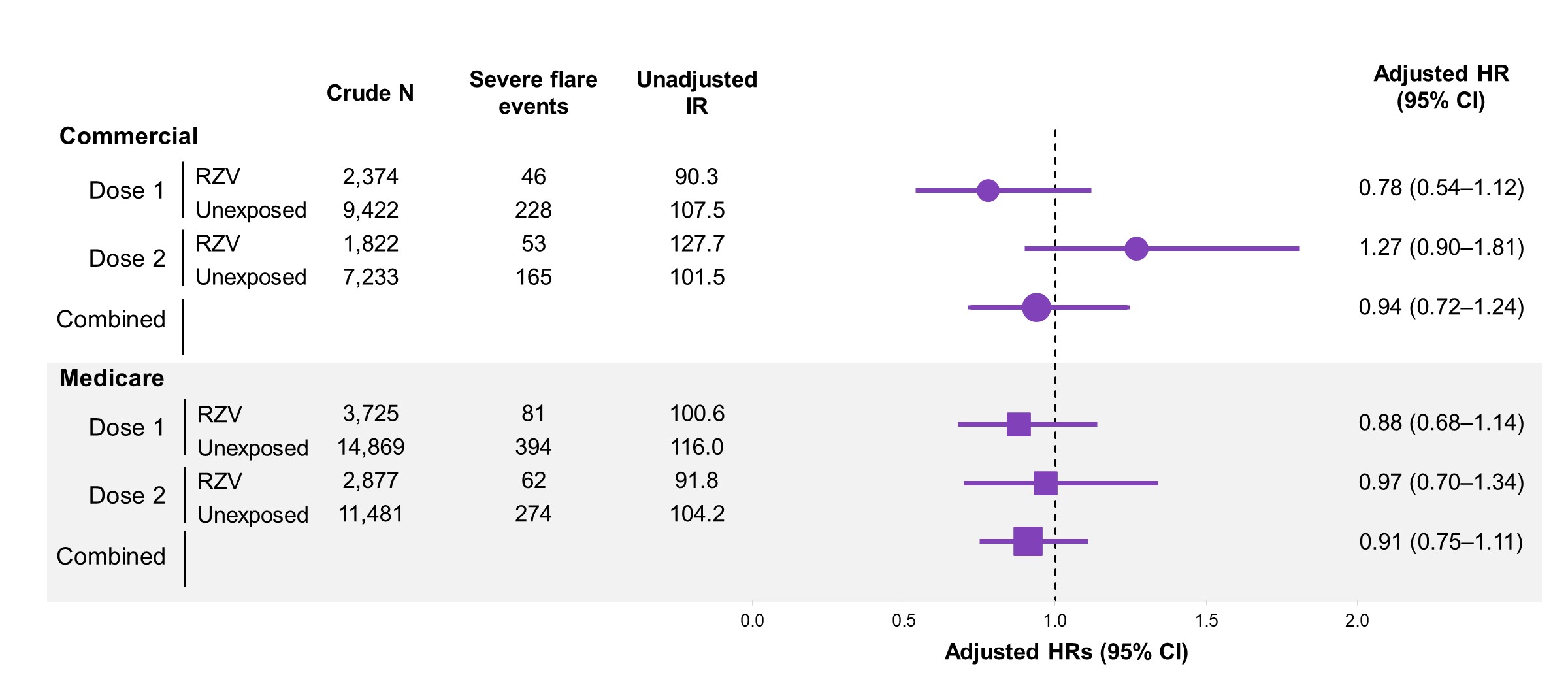
Vaccinated patients had more immunosuppressive use and fewer comorbidities, emergency department visits, and hospitalizations before weighting (Table 1); all characteristics were balanced after weighting. The VE analytic cohorts included 1,308 and 6,025 commercially insured RZV vaccinated patients with SLE and MS, respectively, and 2,284 and 8,705 RZV vaccinated Medicare patients with SLE and MS. The RZV 2-dose VE in commercially insured patients was 54% (95% CI: 18-74) in SLE and 81% (95% CI: 70-88) in MS. In Medicare patients the VE was 70% (95% CI: 50-82) in SLE and 64% (95% CI: 51-74) in MS (Figure 1). The SLE safety analytic cohort included 2,775 commercially insured patients receiving 4,196 RZV doses and 4,227 Medicare patients receiving 6,602 RZV doses. The HR of severe SLE flare within 90 days following RZV was 0.94 (95% CI: 0.72-1.24) in commercially insured patients, and 0.91 (95% CI: 0.75-1.11) in Medicare patients. Dose-specific analysis showed similar results (Figure 2).
Conclusion: The RZV two-dose VE ranged from 54-81% and RZV was not associated with severe SLE flare. These data justify efforts to increase RZV vaccination among patients with SLE and MS.
To cite this abstract in AMA style:
Kluberg S, E. Mayer S, Spence O, Oraichi D, Seifert H, Ali O, Yun H, L. Simon A, S. Ko J, Hugh C, Her M, Shattuck K, Platt R, Jamal-Allial A, Audrey DJIBO D, Daniels K, Ma Q, N McMahill-Walraven C, P. Ogilvie R, Palmsten K, Selvan M, Ziyadeh N, Ogdie A, George M. Effectiveness and Safety of the Recombinant Zoster Vaccine in Patients ≥18 Years of Age with Systemic Lupus Erythematosus or Multiple Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-the-recombinant-zoster-vaccine-in-patients-%e2%89%a518-years-of-age-with-systemic-lupus-erythematosus-or-multiple-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-the-recombinant-zoster-vaccine-in-patients-%e2%89%a518-years-of-age-with-systemic-lupus-erythematosus-or-multiple-sclerosis/