Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The Canada-Denmark (CANDEN) MRI scoring system enables detailed anatomy-based evaluation of inflammatory and structural lesions in spinal vertebral bodies and posterior elements in patients (pts) with AS.1 Tofacitinib is an oral Janus kinase inhibitor under investigation for the treatment of adult pts with AS. In a Phase (P)2 dose-ranging study (NCT01786668), tofacitinib showed greater efficacy vs placebo (PBO) in reducing signs, symptoms, and inflammation based on Spondyloarthritis Research Consortium of Canada MRI SI joint and spine scores in biologic-naïve pts with AS.2 This post hoc analysis evaluated the efficacy of tofacitinib on MRI outcomes for spinal vertebral body and posterolateral element inflammation and structural lesions in pts with AS, assessed with the CANDEN MRI scoring system.
Methods: Biologic-naïve adult pts with active AS (per modified New York criteria) were randomized 1:1:1:1 to tofacitinib 2, 5, or 10 mg twice daily (BID), or PBO in a 16-week (12-week treatment; 4-week follow-up), double-blind P2 study. Spine MRI assessments were performed at baseline (BL) and Week (W)12. This post hoc analysis comprised data from re-evaluation of MRI images in pts in the tofacitinib 5 or 10 mg BID, or PBO groups. Images were read pairwise (both time points displayed simultaneously) by 2 independent readers blinded to time point/treatment, and scored using the CANDEN MRI scoring system. In cases of substantial disagreements between readers, a third reader adjudicated. Least squares mean changes from BL to W12 were reported for CANDEN-specific MRI outcomes, including total spine inflammation score and subscores, and total spine fat score. An analysis of covariance was used for the primary comparison of pooled tofacitinib 5 and 10 mg BID groups vs PBO and for comparisons between tofacitinib 5 or 10 mg BID groups vs PBO. P values without adjustment to multiplicity are reported.
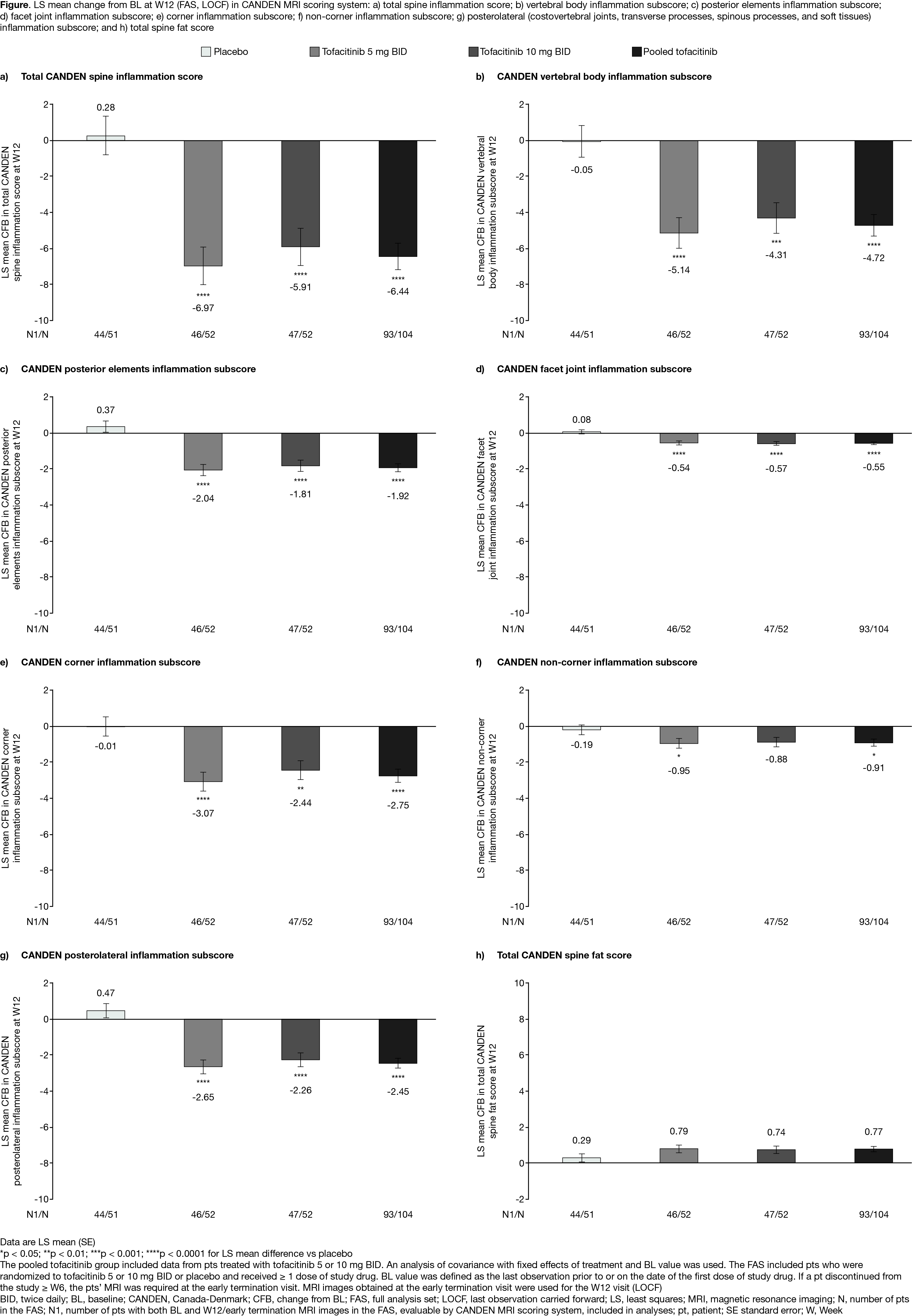
Results: MRI data for 137 pts were evaluated. At W12, significantly greater reductions were seen in the pooled tofacitinib group vs PBO for total CANDEN spine inflammation score (p < 0.0001; Fig a), and CANDEN vertebral body (p < 0.0001; Fig b), posterior elements (p < 0.0001; Fig c), facet joint (p < 0.0001; Fig d), corner (p < 0.0001; Fig e), non-corner (p=0.0310; Fig f), and posterolateral (costovertebral joints, transverse processes, spinous processes, and soft tissues; p < 0.0001; Fig g) inflammation subscores. Similar reductions were seen for these outcomes in the tofacitinib 5 or 10 mg BID groups vs PBO. Regarding fat infiltration, a numerically greater increase in total spine fat score was seen in the pooled tofacitinib group vs placebo (Fig h).
Conclusion: In biologic-naïve pts with AS, tofacitinib treatment was associated with significant reductions in MRI spinal inflammation, as assessed by the CANDEN MRI scoring system. Tofacitinib reduced inflammation in the posterolateral elements of the spine and the facet joints, which has not been described previously.
1. Krabbe S et al. RMD Open 2019; 5: e001057.
2. van der Heijde D et al. Ann Rhem Dis 2017; 76: 1340-7.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by J Arnold, CMC Connect, funded by Pfizer Inc.
To cite this abstract in AMA style:
Ostergaard M, Wu J, Fallon L, Sherlock S, Wang C, Fleishaker D, Kanik K, Maksymowych W. Effect of Tofacitinib on Spinal Vertebral Body and Posterolateral Element Inflammation and Structural Lesions Using the Canada-Denmark MRI Scoring System in Patients with Ankylosing Spondylitis: Results from a Phase 2 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-tofacitinib-on-spinal-vertebral-body-and-posterolateral-element-inflammation-and-structural-lesions-using-the-canada-denmark-mri-scoring-system-in-patients-with-ankylosing-spondylitis-resul/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-tofacitinib-on-spinal-vertebral-body-and-posterolateral-element-inflammation-and-structural-lesions-using-the-canada-denmark-mri-scoring-system-in-patients-with-ankylosing-spondylitis-resul/