Session Information
Date: Sunday, November 13, 2022
Title: Osteoporosis and Metabolic Bone Disease – Basic and Clinical Science Poster
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Osteoarthritis (OA) and osteoporosis (OP) often occur concomitantly in the elderly. The relationship between OA and OP is complex, with increased fracture risk reported to be associated with OA (Wright J Rheumatol 2011). Romosozumab (Romo) is a bone-forming agent approved for OP treatment. Romo binds to and inhibits sclerostin, widely viewed as an osteocyte-specific protein but is also expressed in articular chondrocytes. The expression of sclerostin is focally increased in cartilage in OA while being decreased in the subjacent subchondral bone (Chan Osteoarthritis Cartilage 2011). In FRAME, women with OP were treated with Romo vs placebo (Pbo) for 12 months followed by denosumab for 12 months. Romo led to large BMD improvements and fracture risk reduction within 12 months vs Pbo (Cosman NEJM 2016). FRAME included a prespecified Knee OA Substudy to assess the effect of treatment with Romo for 12 months compared with Pbo on the progression of OA of the knee in women with OP and knee OA. Here we report changes in knee pain, physical function, worsening of knee OA, and treatment-emergent adverse events (AEs) of OA in the Knee OA Substudy.
Methods: Inclusion criteria for the Knee OA Substudy were signal knee pain due to OA, morning stiffness lasting < 30 mins, knee crepitus, and knee OA confirmed by x-ray within 12 months. Change from baseline through month 12 in the WOMAC score, incidence of worsening knee OA, and treatment-emergent AEs were assessed. WOMAC score changes from baseline were assessed by ANCOVA and incidence of worsening knee OA was assessed by logistic regression, adjusting for age, baseline BMI, and baseline WOMAC total score for both statistical models. All p-values were nominal.
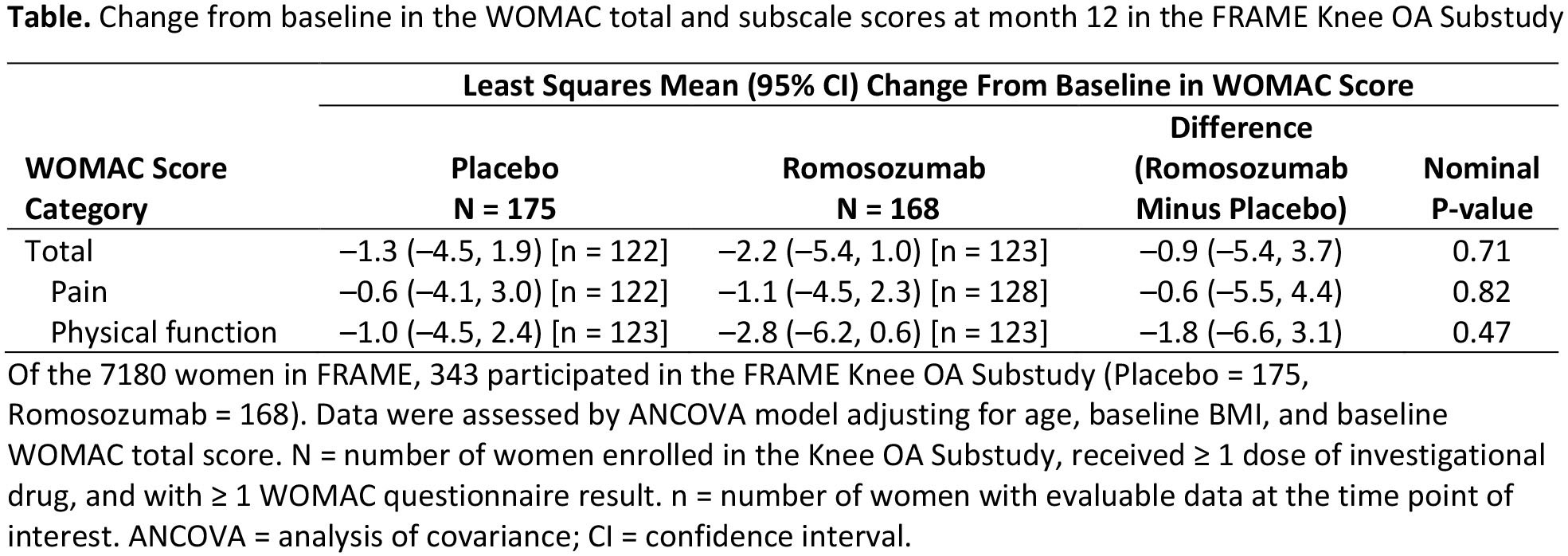
Results: Of 7180 women in FRAME, 343 participated in the Knee OA Substudy (Romo = 168, Pbo = 175). Mean age was 72 years and total WOMAC score was 38. At month 12, no significant difference in progression of knee OA was observed with Romo vs Pbo (least squares mean [95% CI] total WOMAC score: –2.2 [–5.4, 1.0] vs –1.3 [–4.5, 1.9]; P = 0.71; Table). Incidence of worsening symptoms of knee OA was comparable between Romo (17.1%) and Pbo (20.5%), with an odds ratio of 0.9 (95% CI: 0.5, 1.7; P = 0.69). The number of women reporting treatment‑emergent AEs of OA was 13 (7.7%) with Romo and 21 (12.0%) with Pbo.
Conclusion: Romo therapy did not negatively impact knee OA pain or function in postmenopausal women with OP.
To cite this abstract in AMA style:
Lane N, Betah D, Deignan C, Oates M, Wang Z, Timoshenko J, Khan A, Binkley N. Effect of Romosozumab in Postmenopausal Women with Knee Osteoarthritis: Results from the FRAME Clinical Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effect-of-romosozumab-in-postmenopausal-women-with-knee-osteoarthritis-results-from-the-frame-clinical-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-romosozumab-in-postmenopausal-women-with-knee-osteoarthritis-results-from-the-frame-clinical-trial/