Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: RA patients (pts) often suffer substantial pain despite ongoing treatment (tx) and regard pain control as a top tx goal. Filgotinib (FIL)—a potent, oral Janus kinase-1 selective inhibitor—was efficacious and generally well tolerated in the FINCH phase 3 RA clinical trial program.1,2 A post hoc analysis of FINCH studies was conducted to assess the impact of FIL on pain.
Methods: All pts met 2010 ACR/EULAR criteria for RA. In FINCH 3 (F3), MTX-naïve RA pts received FIL 200 mg + MTX, FIL 100 mg + MTX, FIL 200 mg monotherapy, or MTX monotherapy for up to 52 weeks (W). Pts in FINCH 1 (F1) had active RA and inadequate response to MTX (MTX-IR) and received FIL 200 mg, FIL 100 mg, adalimumab (ADA) 40 mg, or placebo (PBO) on a background of MTX for up to 52W; at W24, PBO pts were rerandomized to FIL 200 or 100 mg. In FINCH 2 (F2), pts receiving conventional synthetic (cs)DMARDs who had an inadequate response or intolerance to biologic DMARD (bDMARD-IR) received FIL 200 mg, 100 mg, or PBO on a background dose of csDMARD(s) for up to 24W.
Each study was analyzed separately. Pt-reported pain was assessed on a visual analog scale (VAS). Proportions of pts achieving thresholds of 30% (defined as “moderate clinically important differences”) and 50% (“substantial improvements”)3 reduction from baseline were analyzed, as were exploratory thresholds of 70% and 90%, and residual VAS pain scores of ≤10/20/40 mm out of 100 mm. P-values were calculated from the logistic regression with tx groups and stratification factors in the model. Comparisons were not adjusted for multiplicity; nominal P values are presented and should be interpreted as exploratory.
Results: Median duration of RA since diagnosis was 0.3–0.4 years (y) in F3, 4.8–5.8y in F1, and 9.8–10.3y in F2. Baseline pain was high among all arms (mean VAS scores of 64–68 mm across studies). Pain improved across pt populations from MTX-naïve to bDMARD-IR during tx. At W2, the percent of pts with pain reduction ≥30%, ≥50%, and residual pain ≤40 mm was significantly greater for all FIL arms compared with PBO (F1/F2) or MTX (F3) (nominal P < 0.05; Table 1). Pain was reduced by ≥90% by W52 in approximately 25% of pts in F1/F3 (Table 1). Except for pts receiving FIL 100 mg in the ≥30% reduction analysis, significantly more bDMARD-IR pts receiving FIL had pain reduction at W24 compared with PBO in all analyses (F2; nominal P < 0.05; Table 1). FIL + MTX significantly reduced pain in MTX-naïve pts vs MTX monotherapy (F3; nominal P < 0.05 for FIL 200 for all measures and time points and for FIL 100 for several measures and time points; Table 1). Overall, more pts with MTX-IR (F1) receiving FIL had greater pain reduction and lower residual pain vs pts receiving ADA, with significant differences noted for FIL 200 (but not FIL 100) for some measures at W2–W30 (nominal P < 0.05; Table 1; Fig 1; Fig 2).
Conclusion: FIL 200 and 100 mg provided rapid, clinically meaningful pain relief among a broad spectrum of RA pts and across several measures. The degree of improvement was substantial for many pts; ≥40% of pts in all studies had a ≥50% reduction in pain.
References
1) Genovese et al. JAMA. 2019;322:315–25.
2) Westhovens et al. Arthritis Rheumatol. 2019;71 [suppl 10]:1606–8.
3) Dworkin et al. J Pain. 2008;9:105–21.
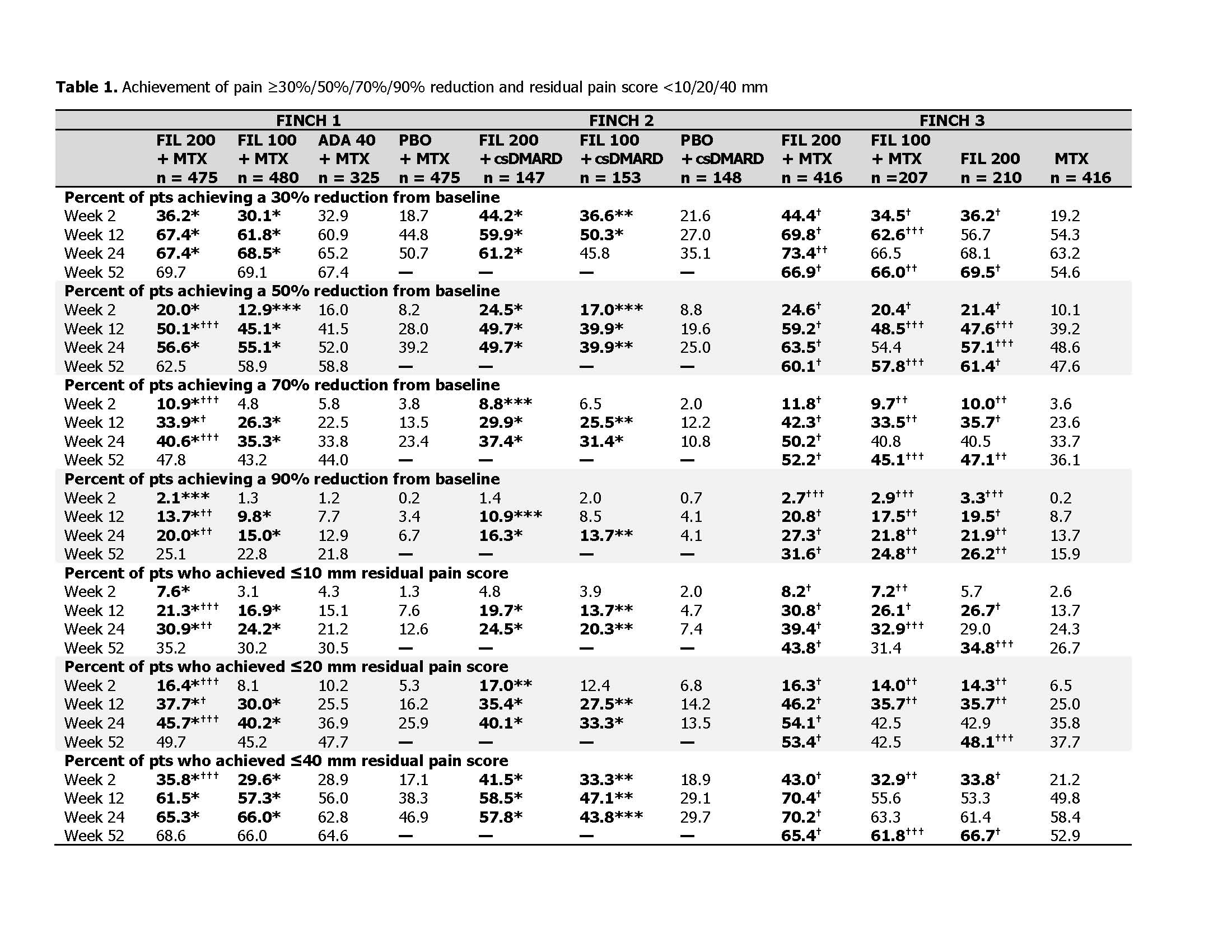 ***Nominal P < 0.05 for FIL arm vs PBO, **nominal P < 0.01 for FIL arm vs PBO, *nominal P < 0.001 for FIL arm vs PBO; †††nominal P < 0.05 for FIL arm vs ADA or MTX, ††nominal P < 0.01 for FIL arm vs ADA or MTX, †nominal P < 0.001 for FIL arm vs ADA or MTX. P-values were calculated from the logistic regression with treatment groups and stratification factors in the model. Pts with missing outcomes were set as nonresponders for binary response measurements. —, not measured at this time point because in FINCH 1, pts receiving PBO were rerandomized 1:1 to FIL 200 or 100 mg at week 24, and, in FINCH 2, the study period ended at 24 weeks. ADA, adalimumab; csDMARD, conventional synthetic DMARD; FIL, filgotinib; PBO, placebo; pts, patients.
***Nominal P < 0.05 for FIL arm vs PBO, **nominal P < 0.01 for FIL arm vs PBO, *nominal P < 0.001 for FIL arm vs PBO; †††nominal P < 0.05 for FIL arm vs ADA or MTX, ††nominal P < 0.01 for FIL arm vs ADA or MTX, †nominal P < 0.001 for FIL arm vs ADA or MTX. P-values were calculated from the logistic regression with treatment groups and stratification factors in the model. Pts with missing outcomes were set as nonresponders for binary response measurements. —, not measured at this time point because in FINCH 1, pts receiving PBO were rerandomized 1:1 to FIL 200 or 100 mg at week 24, and, in FINCH 2, the study period ended at 24 weeks. ADA, adalimumab; csDMARD, conventional synthetic DMARD; FIL, filgotinib; PBO, placebo; pts, patients.
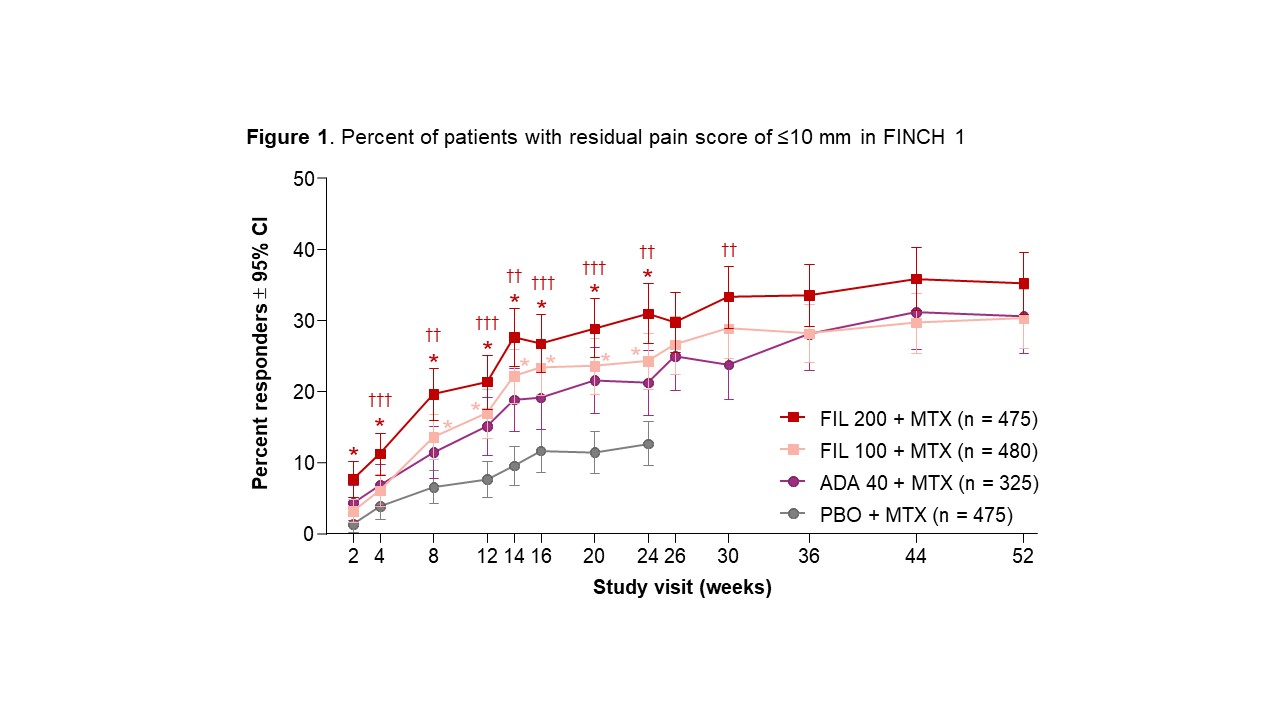 Pts receiving PBO were rerandomized 1:1 to FIL 200 or 100 mg at week 24. P-values for FIL vs PBO were assessed for weeks 2─24; P-values for FIL vs ADA were assessed for all visits. *Nominal P < 0.001 for FIL 200 or FIL 100 vs PBO; †††nominal P < 0.05 for FIL 200 vs ADA, ††nominal P < 0.01 for FIL 200 vs ADA. P-values were calculated from the logistic regression with treatment groups and stratification factors in the model. Pts with missing outcomes were set as nonresponders for binary response measurements. ADA, adalimumab; CI, confidence interval; FIL, filgotinib; PBO, placebo; pts, patients.
Pts receiving PBO were rerandomized 1:1 to FIL 200 or 100 mg at week 24. P-values for FIL vs PBO were assessed for weeks 2─24; P-values for FIL vs ADA were assessed for all visits. *Nominal P < 0.001 for FIL 200 or FIL 100 vs PBO; †††nominal P < 0.05 for FIL 200 vs ADA, ††nominal P < 0.01 for FIL 200 vs ADA. P-values were calculated from the logistic regression with treatment groups and stratification factors in the model. Pts with missing outcomes were set as nonresponders for binary response measurements. ADA, adalimumab; CI, confidence interval; FIL, filgotinib; PBO, placebo; pts, patients.
 Pts receiving PBO were rerandomized 1:1 to FIL 200 or 100 mg at week 24. P-values for FIL vs PBO were assessed for weeks 2–24; P-values for FIL vs ADA were assessed for all visits. ***Nominal P < 0.05 for FIL 100 vs PBO, **nominal P < 0.01 for FIL 100 vs PBO, *nominal P < 0.001 for FIL 200 or FIL 100 vs PBO; †††nominal P < 0.05 for FIL 200 vs ADA, ††nominal P < 0.01 for FIL 200 vs ADA. P-values were calculated from the logistic regression with treatment groups and stratification factors in the model. Pts with missing outcomes were set as nonresponders for binary response measurements. ADA, adalimumab; CI, confidence interval; FIL, filgotinib; PBO, placebo; pts, patients.
Pts receiving PBO were rerandomized 1:1 to FIL 200 or 100 mg at week 24. P-values for FIL vs PBO were assessed for weeks 2–24; P-values for FIL vs ADA were assessed for all visits. ***Nominal P < 0.05 for FIL 100 vs PBO, **nominal P < 0.01 for FIL 100 vs PBO, *nominal P < 0.001 for FIL 200 or FIL 100 vs PBO; †††nominal P < 0.05 for FIL 200 vs ADA, ††nominal P < 0.01 for FIL 200 vs ADA. P-values were calculated from the logistic regression with treatment groups and stratification factors in the model. Pts with missing outcomes were set as nonresponders for binary response measurements. ADA, adalimumab; CI, confidence interval; FIL, filgotinib; PBO, placebo; pts, patients.
To cite this abstract in AMA style:
Taylor P, Kavanaugh A, Nash P, Pope J, Bartok B, Hasegawa K, Rao S, Strengholt S, Westhovens R. Effect of Filgotinib on Pain in Patients with Rheumatoid Arthritis: Results from Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effect-of-filgotinib-on-pain-in-patients-with-rheumatoid-arthritis-results-from-phase-3-clinical-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-filgotinib-on-pain-in-patients-with-rheumatoid-arthritis-results-from-phase-3-clinical-trials/
